In recent years, advancements in the field of neuroscience and neurotechnology have offered groundbreaking pathways for treating complex neurological disorders. Among these innovations, transcranial focused ultrasound (tFUS) has emerged as a non-invasive approach to modulating brain activity through directed high-frequency sound waves. This technology shows particular promise for patients suffering from drug-resistant epilepsy and tremor-related conditions. A dynamic partnership between researchers from Sungkyunkwan University, the Institute for Basic Science, and the Korea Institute of Science and Technology has led to the development of a pioneering brain sensor that enhances the efficacy of tFUS, as documented in their recent publication in *Nature Electronics*.
Historically, the challenge in using sensors for directly monitoring brain activity has been their inability to closely conform to the brain’s intricate structure. Previous iterations of brain sensors, as noted by leading researchers such as Donghee Son, encountered issues with capturing accurate neural signals. These challenges arose particularly in regions characterized by severe curvature, where existing sensors would detach or collect imprecise data.
While a more advanced sensor developed by Professors John A. Rogers and Dae-Hyeong Kim made strides in accessing complex brain surfaces, it still grappled with adherence issues, especially in highly contoured regions. The micro-motions caused by the brain’s natural movements and the flow of cerebrospinal fluid contributed to an unstable connection that complicated consistent data collection. Consequently, there remained a crucial need for a more reliable instrument capable of long-term applications in clinical settings.
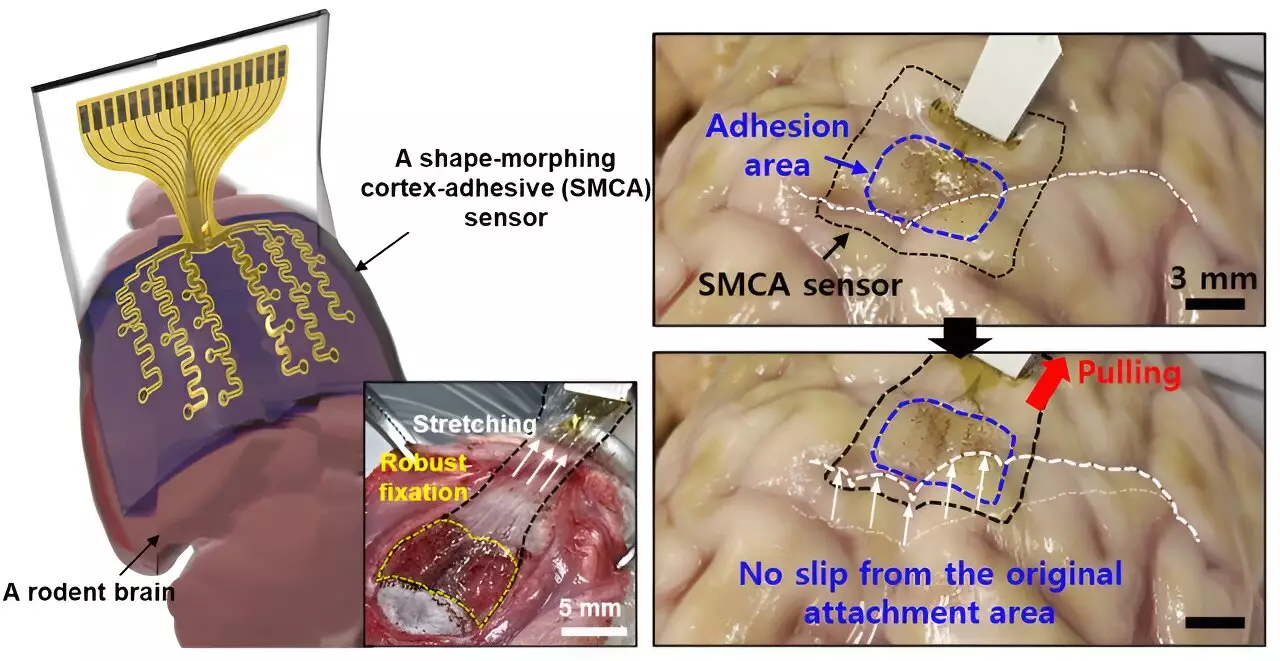
The new sensor, coined the electrocorticography (ECoG) sensor, aims to address these critical limitations by securely adhering to the contours of the brain. What sets this innovation apart is its dynamic structural design that eliminates voids between the sensor and brain tissue, consequently minimizing external mechanical interference that often disrupts signal clarity. As explained by Son, the ECoG sensor exhibits strong adhesion capabilities that significantly improve the precision of data gathered from targeted brain areas, especially for therapies aimed at curbing epileptic episodes.
This shape-adaptive feature allows the sensor to maintain a consistent contact area with the highly variable surface of the brain, facilitating uninterrupted monitoring of neural activity. Moreover, the implications extend well beyond epilepsy management; the development paves the way for tailored treatment solutions for a variety of neurological disorders.
A highlight of this technology is its potential for contributing to personalized medicine strategies. To optimize treatments, it is crucial for medical professionals to analyze brain wave patterns in real-time while simultaneously delivering ultrasound stimulation to specific regions. Traditional sensors have struggled with this dual functionality due to the signal noise generated by ultrasound vibrations, complicating the adjustment of treatment modalities according to individual patient profiles.
Son and his team have crafted a solution that not only reduces this noise but enhances the fidelity of brain signal acquisition. The ECoG sensor leverages a three-layer structure: a hydrogel layer for tissue bonding, a self-healing polymer layer that can morph to the required shape, and a stretchable ultra-thin electrode layer for effective signaling. Collectively, these elements facilitate seamless operations, providing valuable real-time insights into each patient’s unique brain activity.
Early trials conducted on awake rodent models have shown remarkable results, allowing the research team to effectively monitor brain waves and manage induced seizures. These initial successes provide an optimistic outlook for the future of this technology. The researchers aim to refine the sensor further by developing a high-density electrode array to enhance the resolution and scope of brain signal mapping.
Planning for clinical trials and scaling the technology are critical next steps for the researchers to bring this innovation closer to practical use. With the ultimate goal of revolutionizing treatment methodologies not only for epilepsy but also for a wide range of brain disorders, the ECoG sensor represents a significant leap in neurotechnology.
The advancements brought forth by the development of this adaptable brain sensor may herald a new era in the treatment of neurological conditions. As researchers continue to fine-tune its capabilities and assess its effectiveness in clinical environments, we inch closer to a future where personalized solutions can significantly improve the quality of life for patients grappling with debilitating disorders. Indeed, the combination of innovative design and real-time adaptability holds the key to unlocking new frontiers in neurotherapeutics and enhancing our understanding of the complexities of the human brain.

Leave a Reply