As the world grapples with the ever-evolving challenges of climate change, carbon dioxide (CO2) emissions are consistently recognized as a principal contributor to global warming. Not only does CO2 pose dire risks to the environment, but it also threatens ecosystems, public health, and economic stability. With these pressing issues at hand, innovative solutions must be explored to mitigate these emissions. One emerging method lies in the potential of cement-based materials to help capture and mineralize CO2 through a process known as carbonation. This not only opens up new avenues for construction practices but also introduces a win-win scenario where building materials can aid in the fight against climate change.
The carbonation process in cement paste is intricate. It primarily involves the dissolution of CO2 in water, which subsequently interacts with compounds known as calcium silicate hydrates (C–S–H). These compounds result from the hydration of raw cement materials. The dissolved CO2 reacts to form carbonate ions (CO32-), which further interact with calcium ions (Ca2+) originating from the C–S–H structure, leading to the formation of calcium carbonate precipitates. However, despite a plethora of research devoted to understanding this process, a comprehensive understanding is still lacking. The complexity arises from the unstable nature of the compounds involved in cement paste, which makes it a challenging subject to study.
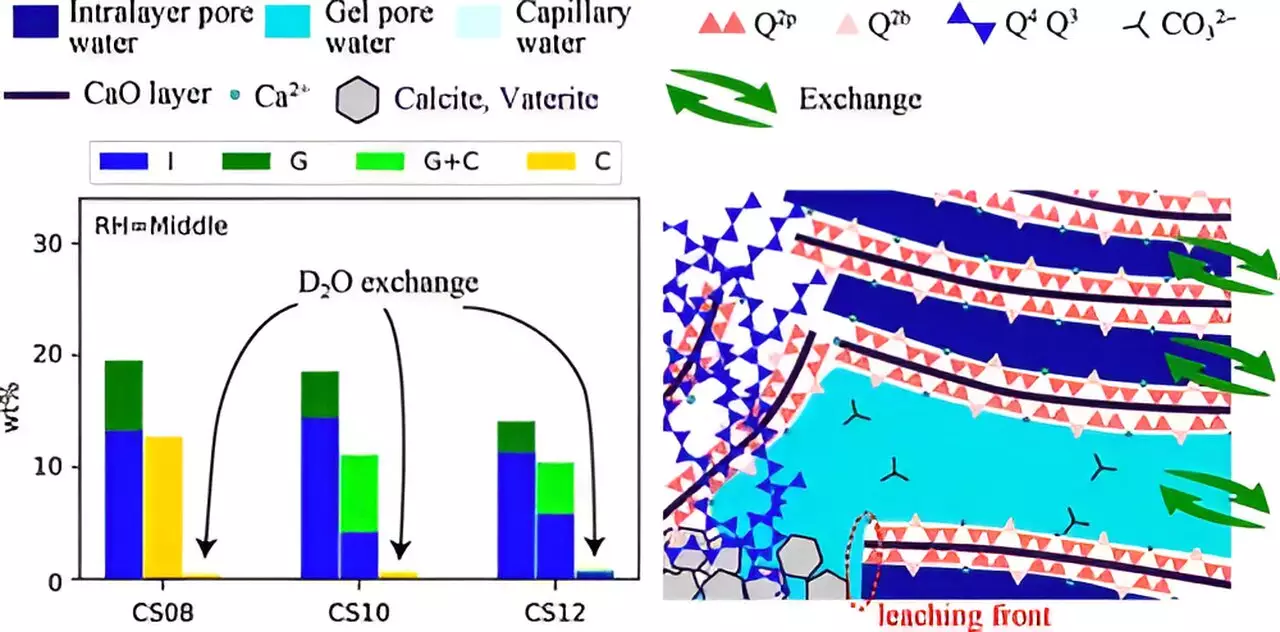
Multiple factors affect the efficiency of carbonation, such as the relative humidity (RH) of the environment, CO2 solubility, and the calcium/silicate (Ca/Si) ratio present within the C–S–H framework. Additionally, the ion transport and water movement within the nanometer-sized pores of C–S–H are critical, particularly concerning an environment referred to as gel-pore water. These variables create a multifaceted landscape that researchers need to navigate to unlock the mechanism behind carbonation.
In a recent study led by Associate Professor Takahiro Ohkubo and his team from Chiba University, the complexities of carbonation reactions were put under the microscope. They unveiled the lesser-explored aspects of water transport and structural changes associated with carbonation, utilizing advanced techniques such as 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry. These methods have proven effective in studying water dynamics within the C–S–H phases, providing valuable insights that were previously shadowed.
One standout feature of this ground-breaking research is the implementation of accelerated carbonation experiments. Traditional carbonation occurs over prolonged periods, often spanning decades, when CO2 is absorbed from the atmosphere. However, experiments conducted with significantly elevated CO2 concentrations allow researchers to fast-track this process. By synthesizing the C–S–H under various RH conditions and Ca/Si ratios, the team was able to closely examine how these factors influenced structural changes during carbonation.
The findings revealed that carbonation significantly impacted the structural integrity of the C–S–H chain—specifically, changes in pore sizes and chain collapse were noted. Lower RH levels, when combined with high Ca/Si ratios, resulted in smaller pore sizes while hindering the leaching of Ca2+ ions. This obstruction, in turn, resulted in less efficient carbonation due to the limited movement of water and ions within the material.
A pivotal takeaway from this research is the importance of understanding not just structural changes but also the mass transfer properties that govern the carbonation reaction. The interplay between these two facets is pivotal for enhancing the carbonation efficiency of cement-based materials. Professor Ohkubo emphasizes that examining this delicate balance is crucial for future studies aimed at developing more efficient materials.
Looking forward, the implications of these findings are immense. By refining the carbonation process in cement-based materials, researchers can play a crucial role in creating building materials with a higher capacity to absorb atmospheric CO2. This presents a substantial opportunity for the construction industry, which has long been criticized for its environmental footprint.
Furthermore, the methodologies developed in this study could extend beyond construction applications. The mechanisms of carbonation could also be applied to understand organic materials in natural environments, enhancing our broader comprehension of carbon capture in various ecosystems.
As CO2 emissions continue to escalate, the quest for solutions is more urgent than ever. This pioneering research on the carbonation mechanisms in cement-based materials stands as a beacon of hope in the battle against climate change. By elucidating the intricacies of the carbonation process and opening avenues for the development of carbon-absorbing materials, we may be able to take meaningful steps toward a more sustainable future. The intersection of innovative research and practical application has never been more critical in addressing one of humanity’s most formidable challenges.

Leave a Reply