As the world grapples with the impending consequences of climate change and energy shortages, hydrogen energy has emerged as a beacon of hope in the quest for renewable energy sources. With its zero-emission profile and high calorific value, hydrogen has the potential to revolutionize how we approach energy conversion and storage. Among the most promising techniques for hydrogen production is electrochemical water splitting, which allows for efficient hydrogen generation through the dissociation of water molecules into hydrogen and oxygen. However, this method encounters significant challenges, primarily due to the sluggish kinetics associated with the oxygen evolution reaction (OER), which occurs at the anode. Therefore, advancing catalyst effectiveness is essential for making hydrogen production through water splitting a viable and efficient option.
The main bottleneck in electrochemical water splitting is the low efficiency of the OER, primarily because of the slow kinetics on which it relies. This is where the development of catalysts becomes paramount. Catalysts, which speed up chemical reactions without being consumed in the process, play a crucial role in enhancing the reaction rates of both hydrogen evolution and oxygen evolution reactions. Although a variety of catalysts exist, most struggle with stability and efficiency under operational conditions, necessitating innovative approaches to catalyst design. Recent research emphasizes single-atom catalysts (SACs) and their potential to optimize OER performance by increasing the surface density of active sites, facilitating interactions among neighboring atoms and improving catalytic outcomes.
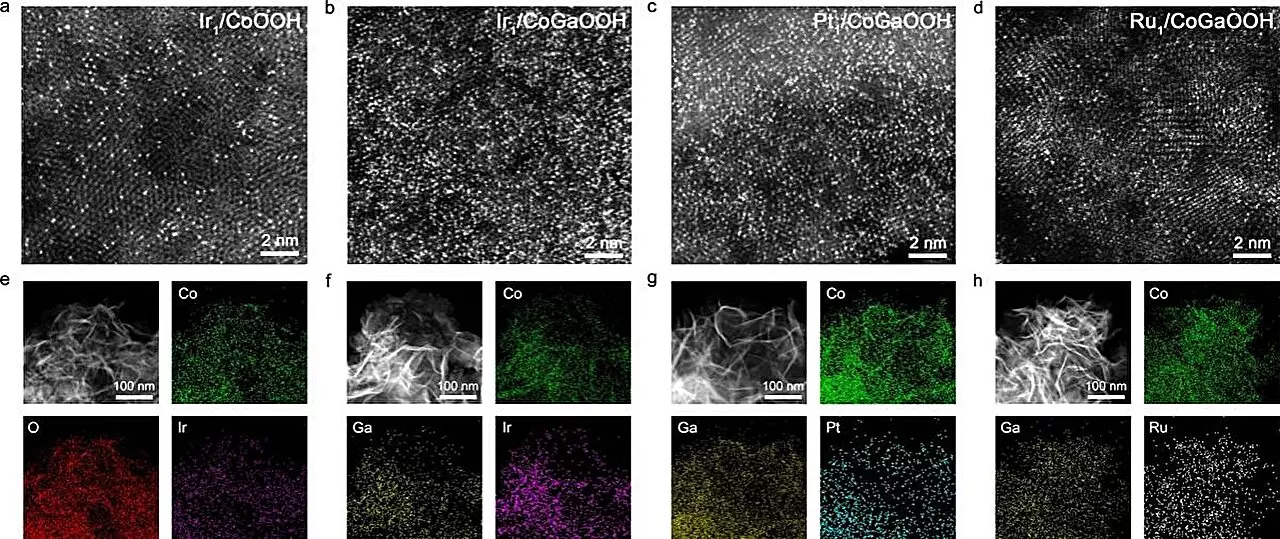
Recent advancements have showcased the potential of high-density single-atom catalysts in addressing the efficiency hurdles associated with the OER. For instance, a research project spearheaded by Prof. Bao Jun at the University of Science and Technology of China (USTC) has made significant strides in this domain. The team explored cobalt-based oxide-supported iridium (Ir) single-atom catalysts and discovered remarkable improvements in OER performance linked to the synergetic relationships formed between closely situated single atoms. These relations enhance the adsorption characteristics of reaction intermediates, thereby optimizing the kinetics of the overall reaction.
To maximize the effectiveness of these single atom catalysts, the USTC team innovatively introduced gallium (Ga) atoms into the cobalt oxide lattice. This strategic manipulation of the electronic structure allowed them to effectively stabilize a high density of Ir single atoms on the catalyst’s surface. By doing so, they successfully achieved a robust catalyst capable of displaying excellent OER performance metrics.
The team’s examination of the catalyst, denoted as Nei-Ir1/CoGaOOH, yielded compelling results. Not only did it demonstrate a strikingly low overpotential of 170 mV at a current density of 10 mA cm⁻², but it also displayed remarkable stability, maintaining effectiveness for over 2000 hours. Additionally, under alkaline electrolyte conditions, it proved capable of functioning stably at 1 A cm⁻² for more than 50 hours, marking a significant achievement in catalyst durability.
Raman spectroscopy further corroborated the catalyst’s structural integrity throughout the OER, affirming its reliability in demanding conditions. The researchers also delved into the mechanism behind the observed enhancements, revealing that the key factors driving performance were not merely electronic structural optimizations but rather the neighboring synergetic interactions among the high-density Ir atoms. These interactions effectively stabilize the *OOH intermediates via hydrogen bonding, substantially reducing the energy barriers for the reaction.
With the findings published in the prestigious journal Angewandte Chemie, this research opens new avenues for the future of OER catalysts. By unlocking the potential of high-density single-atom catalysts, researchers have established a framework for developing advanced electrochemical systems capable of addressing the pressing challenges of hydrogen production. As we continue to innovate in this field, the promise of hydrogen energy as a sustainable and efficient fuel source becomes increasingly attainable. This work represents not just an academic achievement but a vital step toward realizing a greener, low-carbon economy that relies less on fossil fuels and more on renewable energy alternatives. The journey is ongoing, but the foundation laid by such groundbreaking research brings us closer to a future powered by clean hydrogen energy.

Leave a Reply