With the increasing urgency to transition to more environmentally friendly manufacturing practices, the chemical industry faces significant challenges in producing essential precursors for the synthesis of complex organic compounds. Conventional methods, such as naphtha reforming, are energy-intensive processes that contribute to carbon emissions. The recent research conducted by a team from Kyushu University shines light on an innovative method that could address these challenges: employing microwaves to enhance the chemical conversion efficiency of biomass into olefins, using a specific zeolite material known as Na-ZSM-5.
Zeolites are naturally occurring minerals renowned for their porous structure and catalytic properties. They are integral to various catalytic processes, particularly in upgrading hydrocarbons into more valuable chemicals. The process of catalytic cracking, which employs zeolites, allows for the transformation of inexpensive feedstocks like waste cooking oil and microalgal oils into simpler chemical precursors. However, conventional catalytic cracking operates at extremely high temperatures—often between 500 and 600 degrees Celsius—resulting in high energy consumption and other challenges, such as coking. Coking not only decreases the catalyst’s lifespan but also complicates the operation by creating unwanted deposits.
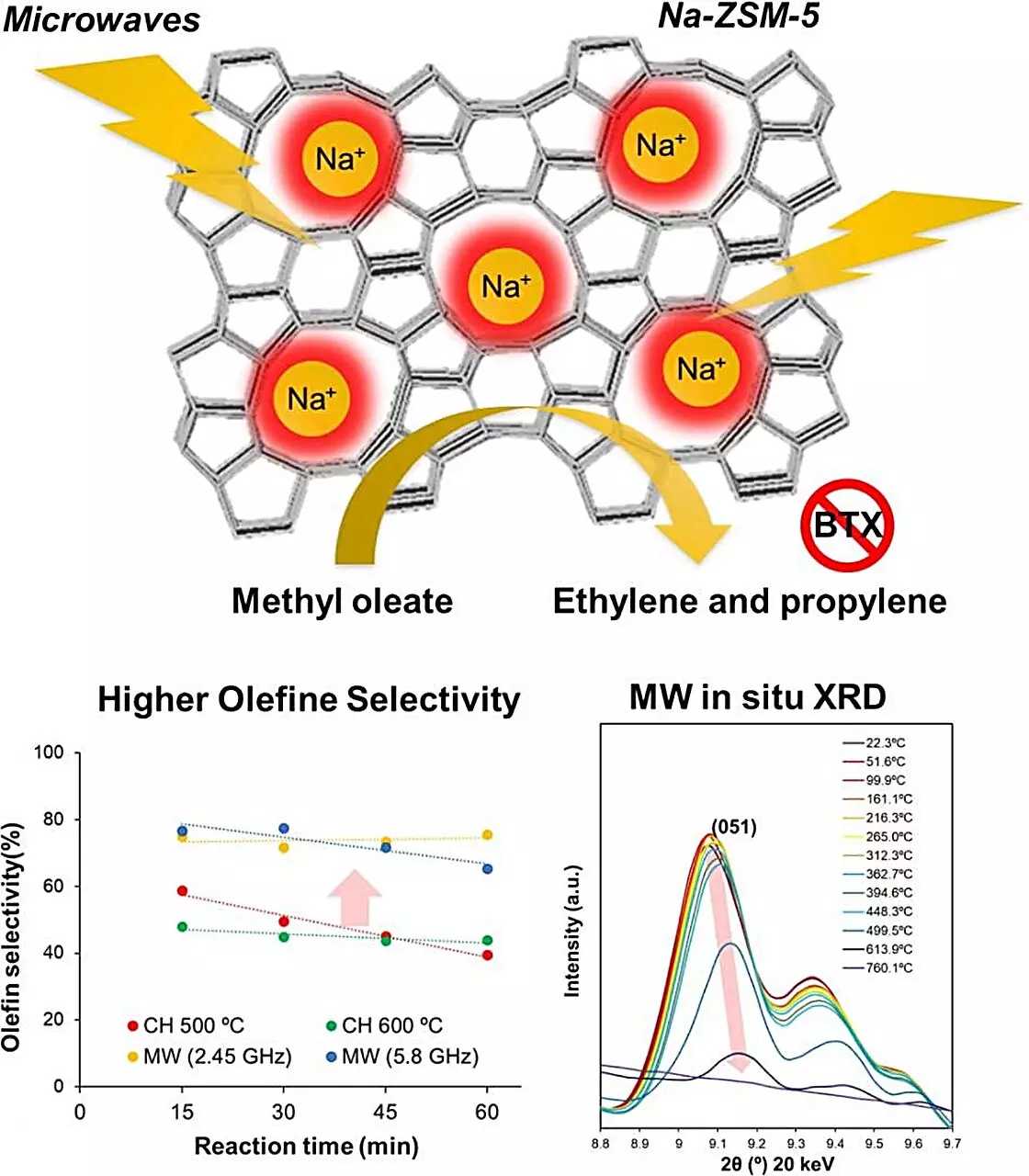
In this context, the Kyushu University team’s exploration of microwave-assisted heating presents a pivotal shift. By utilizing microwaves, the researchers aimed to heat Na-ZSM-5 efficiently while minimizing coking and enhancing overall catalytic performance.
The study, led by Associate Professor Shuntaro Tsubaki, investigates the effects of microwave heating on zeolite catalysts. Microwaves have a unique ability to interact directly with materials, allowing for selective heating. This property enables significant energy savings compared to traditional heat transfer methods, making microwave heating not just a viable alternative but potentially a superior method for biomass conversion.
In their experiments, the researchers compared various zeolite types to discern which exhibited the most effective microwave absorption and catalytic performance. Ultimately, Na-ZSM-5 emerged as the standout candidate, showing remarkable efficiency in converting fatty acid esters into olefins at elevated temperatures. The findings revealed a staggering fourfold increase in olefin production when using microwave heating at 500 degrees Celsius compared to conventional heating methods, with minimal carbon dioxide emissions.
A critical aspect of the research involved delving into the mechanism behind the enhanced catalytic activity observed with microwave heating. The researchers discovered that microwave energy could create localized temperatures exceeding 1000 degrees Celsius within the zeolite crystal lattice, while maintaining a bulk temperature of 500 degrees Celsius. This phenomenon of localized heating is likely responsible for driving the selective formation of olefins, as the extreme conditions enable the breaking of molecular bonds more efficiently and effectively than traditional heating methods.
Additionally, the suppression of coking during microwave processing indicates that not only is the energy efficiency improved, but the long-term viability of the catalyst also receives a boost. By circumventing the coking issue, the team has potentially unlocked new avenues for sustainable chemical manufacturing, paving the way for a more resilient catalyst that can operate under demanding conditions without the typical degradation.
The implications of this research extend well beyond the laboratory. The ability to generate microwaves from renewable energy sources like solar and wind power positions this method as a sustainable alternative for large-scale chemical production. With the chemical industry continually striving to reduce its carbon footprint, embracing microwave-assisted catalytic processes could be pivotal in achieving broader sustainability goals.
Professor Tsubaki articulated a hopeful vision for the future, suggesting that advancements in microwave-driven catalytic processes could enable enhanced yields and energy efficiency while maintaining scalability. If successful, these innovations could usher in a new era of cleaner, more sustainable chemical manufacturing.
The novel research conducted by the Kyushu University team on the use of microwave-assisted Na-ZSM-5 catalysts for biomass conversion holds great promise for transforming the landscape of the chemical industry. As the world grapples with environmental concerns and the need for sustainable practices, the integration of microwaves into chemical processes can lead to significant strides in efficiency and reduction of harmful emissions. With ongoing research and development in this area, the potential for a sustainable chemical revolution appears increasingly plausible, signifying a paradigm shift toward greener industrial practices.

Leave a Reply