Recent research efforts by a collaborative team from the Fritz Haber Institute, Sorbonne University, and Uppsala University have significantly advanced our grasp of ion behavior in aqueous solutions. Their groundbreaking study, published in the prestigious journal *Nature Communications*, introduces innovative methodologies to explore the intricate layers of solvent molecules, known as solvation shells, that form when substances dissolve in liquid. These findings promise to enrich our understanding of chemical interactions and aid in various scientific domains.
The solvation shell is crucial because it encapsulates the interactions between solvent molecules and dissolved ions or molecules. This complicates their properties, as the solvent molecules within this shell behave differently compared to those freely moving in the solution. Consequently, probing these shells has presented a formidable challenge for researchers, primarily due to their evasive and dynamic nature. Traditional methods often fail to isolate or represent these specific interactions comprehensively.
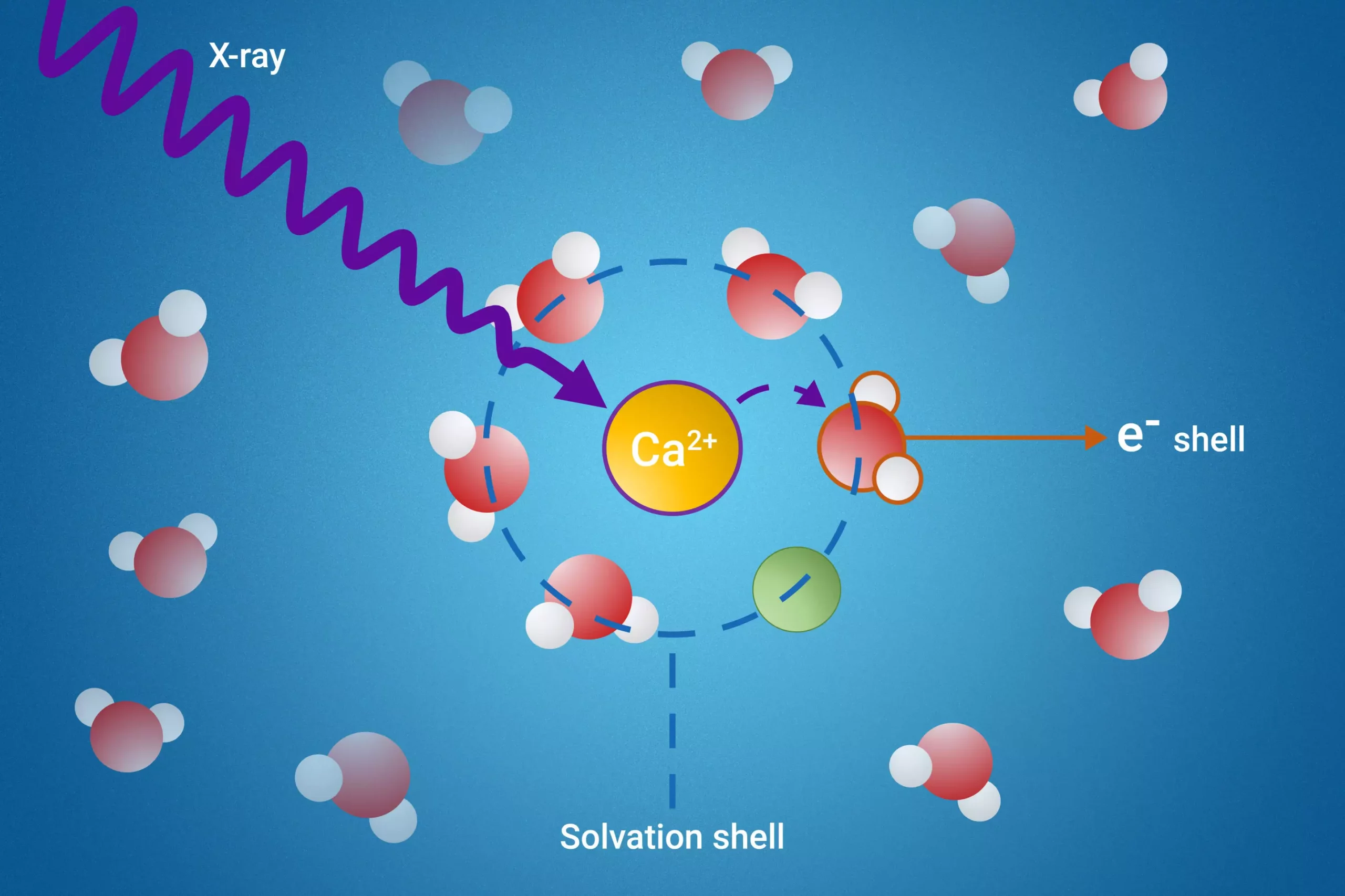
The researchers have successfully devised a novel approach utilizing resonant intermolecular Coulombic decay (ICD), a process that enables them to investigate the solvation shell with remarkable precision. By employing X-ray excitation, the team can observe how solvated molecules interact during the decay process, allowing them to gain unprecedented insights into these molecular layers. This innovative technique not only enhances the understanding of solvation shells but also identifies specific interactions that unfold between solvent and solute.
A key revelation from the research highlights the direct correlation between the ICD process and ion pair formations. The ability to measure the electron binding energies of water molecules within the first solvation shell marks a milestone in solvation studies, as previous methodologies lacked the capability to achieve such detailed measurements. This refined understanding of the solvation shell dynamics can open doors to enhanced molecular design strategies in fields like materials science and electrochemistry.
The implications of this study extend across multiple scientific arenas. A deeper comprehension of solvation shells is paramount in chemistry for predicting reaction mechanisms and solvent effects on chemical processes. In biology, understanding how solvation shells influence biomolecular interactions can impact drug design and enzymatic function. Additionally, advancements in atmospheric science and materials engineering hinge on the minute details of solvation interactions.
The innovative methodology described in this research represents a significant step forward. By enabling precise studies of solvation dynamics, scientists are now better equipped to unravel complex interactions at the molecular level. This not only transforms our understanding of solvation but also holds potential for advancing the efficiency of chemical reactions and materials innovations.
The integrated techniques outlined by the research team signify a promising advancement in the field of solution chemistry. Their work firmly establishes a foundation for future studies on solvation shells, with expansive implications for both theoretical and applied sciences. As we continue to probe deeper into molecular behaviors, the derived insights from such studies will undoubtedly inspire new strategies and applications across diverse scientific disciplines.

Leave a Reply