In a groundbreaking scientific advancement, researchers at the University of Illinois Urbana-Champaign have introduced a remarkable polymer that selectively attracts specific substances from solutions when subjected to electrical activation. This innovative approach marks a significant milestone in the quest for sustainable chemical separation processes. By focusing on the specialized chemical interaction of halogen bonding, the research team has demonstrated a fresh perspective in the design of functional materials that can provide efficient separation methods, representing a shift from traditional practices.
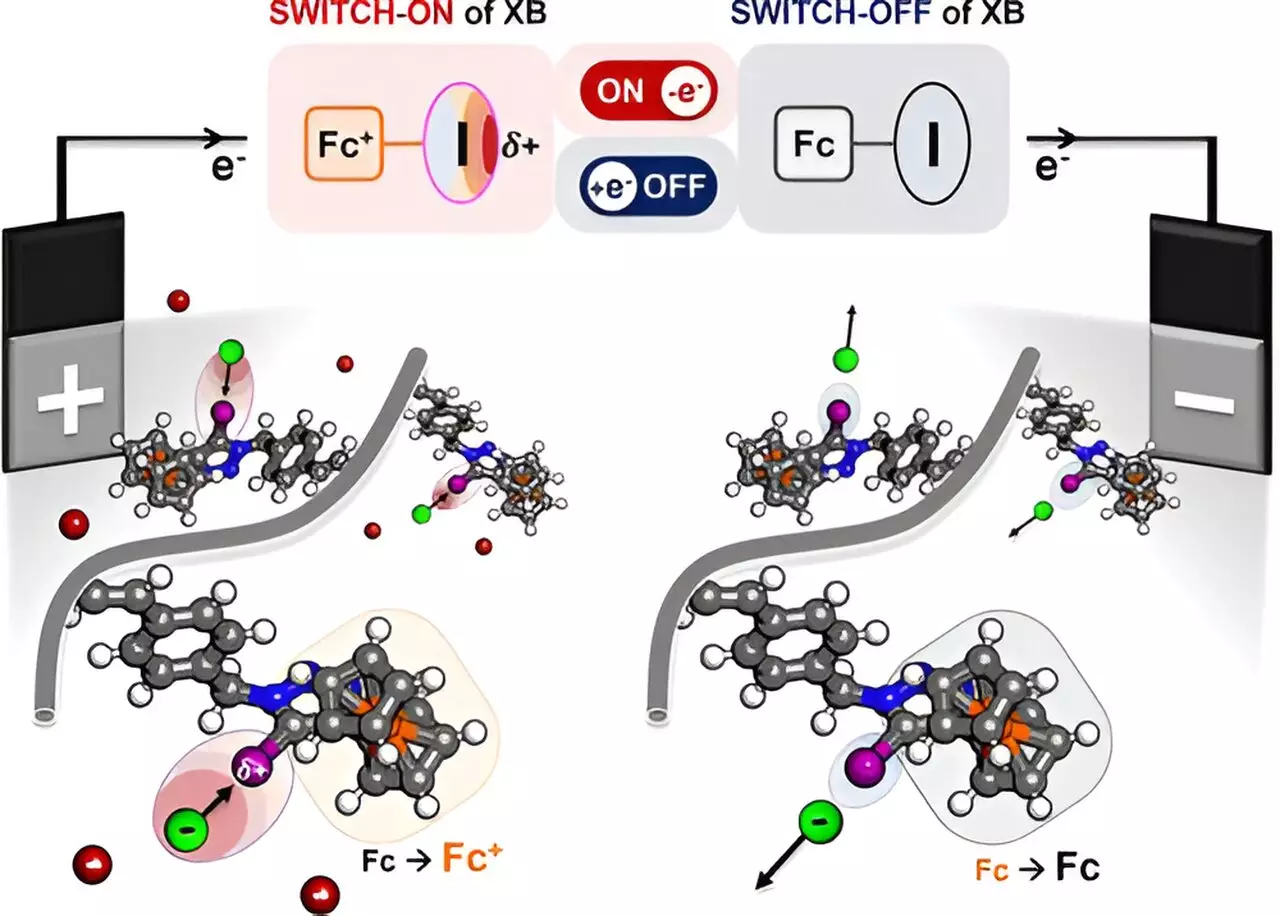
The newly engineered polymer leverages the principle of halogen bonding, a phenomenon where a halogen atom, such as iodine, engages selectively with target molecules. The researchers ingeniously designed the polymer to modulate the charge density of the halogen atom through an applied electrical field. This electrical activation alters the polymer’s attraction capabilities, allowing it to selectively draw in certain target substances—like halides and oxyanions—from a complex mixture. The polymer acts as what the project’s lead, Professor Xiao Su, refers to as an “electric sponge,” thus precisely absorbing only the desired components from a solution.
This unique approach is essential for industries where chemical separation is crucial, such as pharmaceuticals and chemical synthesis. In traditional industrial practices, separation methods often rely on energy-intensive processes like distillation or membrane filtration, which tend to generate a considerable amount of waste. The new electrochemical strategy not only promises efficiency but also minimizes the environmental footprint of chemical manufacturing, heralding a significant transformation towards more sustainable practices.
The research team’s efforts culminated in the successful demonstration of selective electrical separation based on sophisticated molecular interactions. By integrating a redox-active portion—ferrocene—with the halogen-containing polymer, the researchers were able to fine-tune the bonding strength of the iodine atom through electrochemical modulation. When subjected to oxidation, the iodine’s sigma hole activates, creating a strong positive charge that specifically lures negatively charged ions within the solution.
The validation of this innovative approach involved meticulous experimental procedures, including nuclear magnetic resonance (NMR) and Raman scattering analyses, confirming the presence of halogen bonding interactions. Graduate student Nayeong Kim, who played a critical role in the study, emphasized the novelty of applying halogen bonding in a practical application such as this, which was previously explored predominantly in theoretical chemistry.
Though this achievement represents a substantial leap in the field of chemical separation, the researchers acknowledge that further work lies ahead. In the pursuit of industrial application, the team plans to refine and scale this innovative process. Exploring mechanisms such as a cascade model can enhance product purity, while designing a continuous electrosorption system can augment efficiency.
The implications of this research extend beyond mere academic interest; the potential applications are vast. Industries involved in pharmaceuticals, resource recovery, and environmental remediation might benefit tremendously from these advancements. Furthermore, the prospect of employing sustainable energy sources to drive such electrochemical processes could align with broader global goals for reducing reliance on fossil fuels and minimizing environmental impact.
This innovative work by the team at the University of Illinois Urbana-Champaign not only demonstrates the successful application of halogen bonding for targeted molecular interactions but also sets the stage for a new paradigm in chemical separation methodologies. By fostering a deeper understanding of intermolecular forces and leveraging them through electrochemical means, researchers are paving the way towards a future where chemical separations are efficient, selective, and sustainable. As the team looks to future developments, the expansion of this technology could offer transformative benefits across multiple industries, ensuring that the strides made today result in lasting contributions to environmental sustainability and industrial efficiency.

Leave a Reply