In recent years, the push for sustainability in chemistry has become increasingly pressing as industries look for alternatives to harmful practices. Traditional chemical synthesis often relies heavily on toxic organic solvents, generating an alarming amount of waste—over 80% of the total waste produced in chemical processes stems from these solvents. The treatment and disposal of this waste pose significant environmental challenges. To address this issue, researchers at the Indian Institute of Science (IISc) have proposed a groundbreaking approach that leverages agricultural byproducts to reduce the reliance on hazardous materials in chemical reactions.
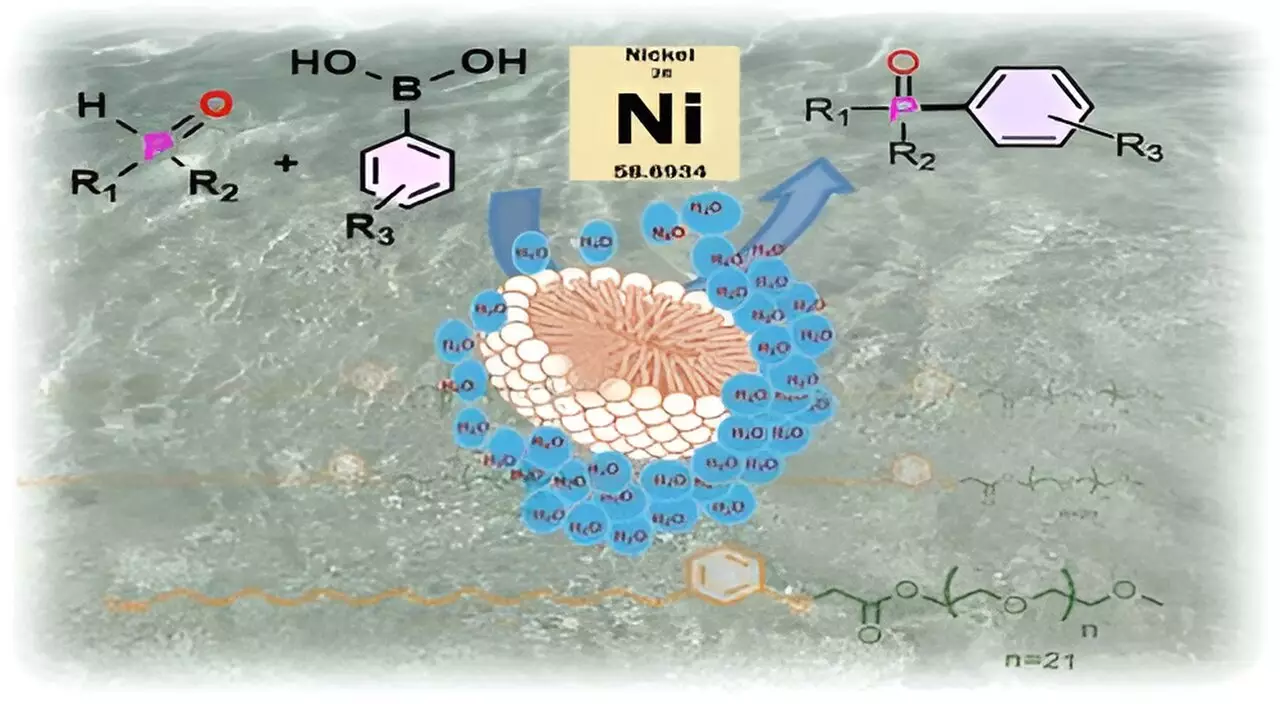
One of the key components of this innovation involves the development of surfactants, which are compounds that can facilitate the interaction of reactants in aqueous solutions. Surfactants possess dual properties—a hydrophilic (water-attracting) part and a hydrophobic (water-repelling) part—allowing them to self-assemble into structures known as micelles. Micelles create isolated environments, facilitating reactions that would otherwise be inhibited due to the sensitivity of certain substrates and catalysts to water.
The IISc research team has synthesized a novel surfactant from cashew nut shell liquid (CNSL), a readily available agricultural waste product. Their surfactant, dubbed CNSL-1000-M, combines cardanol—a significant component derived from CNSL—with m-PEG, a hydrophilic polymer. This innovative combination not only offers a sustainable option for catalysis but also demonstrates the potential of repurposing waste materials in industrial applications.
The micellar catalysis process developed by the IISc team can be likened to a sealed football bobbing on water. The outside of the micelle interacts within the aqueous environment, while the internal hydrophobic pocket remains safe from moisture. Substrates and catalysts, which tend to degrade or react undesirably in the presence of water, can reside within this protected space.
The researchers have illustrated that this technology is not merely theoretical. In practical applications, the CNSL-1000-M surfactant successfully catalyzes the formation of carbon-phosphorus bonds, a vital step in synthesizing numerous essential compounds, including medicinal drugs and organic light-emitting diodes (OLEDs). Remarkably, their findings indicate that the yields of these reactions are significantly enhanced, boasting an 80% increase when performed in water with the new surfactant compared to traditional organic solvents. This not only paves the way for effective reactions at lower temperatures but also allows for the substitution of costly palladium catalysts with more economical nickel alternatives.
This research signals a potential paradigm shift in how industrial chemistry operates, emphasizing the need for greener practices. By utilizing CNSL-1000-M, industries can move toward more responsible chemical synthesis, minimizing environmental hazards associated with organic solvents. The successful demonstration of micellar catalysis prompts further exploration of its capabilities, encouraging researchers and industries alike to delve deeper into this novel chemical approach.
The IISc team, lead by Pritesh Keshari and supported by Susanta Hazra, aims to extend their studies to refine micellar chemistry further and assess its scalability for industrial use. The implications for sustainability are profound; transitioning from toxic solvents to a micellar system based on renewable resources not only conserves materials but also enhances product yields and lowers costs, aligning with the global shift toward green chemistry.
The research conducted by the IISc team marks an important milestone in the quest for sustainable chemical processes. By turning to agricultural waste to develop a new surfactant, they are not just offering a solution to an urgent environmental issue but also demonstrating a versatile approach that integrates science, sustainability, and innovation. As the world increasingly recognizes the need for environmentally friendly practices, advancements like micellar catalysis will play a crucial role in redefining the landscape of the chemical industry, fostering a healthier planet for future generations.

Leave a Reply