The growing demand for efficient and safe energy storage technologies has prompted extensive research into solid-state electrolytes, particularly for their application in solid-state batteries. Compared to conventional liquid electrolytes, these solid alternatives present numerous safety advantages, including reduced flammability and improved stability. Researchers striving to enhance the performance of solid polymer electrolytes face the challenge of developing innovative materials that meet the requirements of next-generation energy systems. Recent findings from a team of materials scientists at the University of Illinois Urbana-Champaign offer insights into how manipulating the molecular structure of these electrolytes can lead to improved conductivity and stability.
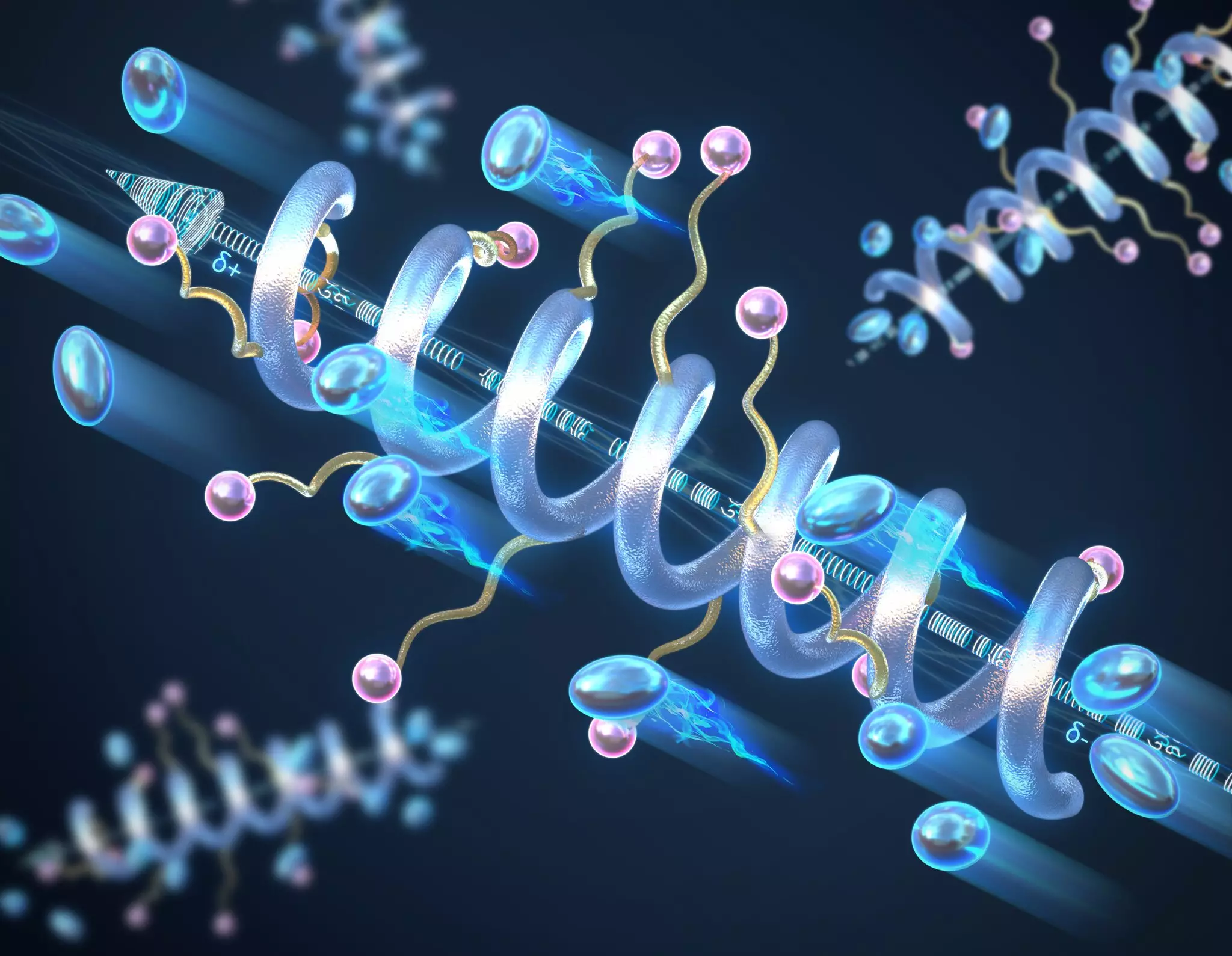
A critical breakthrough highlighted in this study revolves around the incorporation of helical secondary structures in solid-state peptide polymer electrolytes. These helical formations, reminiscent of biological peptides, exhibit significantly better ionic conductivity compared to their non-helical, random coil counterparts. The study’s lead researcher, Professor Chris Evans, emphasizes the revolutionary potential of employing biological principles for materials engineering. By adopting a helical arrangement, the polymer’s conductivity is not only enhanced but also becomes more robust, allowing it to withstand higher temperatures and voltages without compromising its structural integrity.
The concept of helical structures is tied to the macrodipole moment created when polymers assume this specific configuration. This macrodipole results from the cumulative effects of the individual dipole moments within the helical structure. The presence of this macrodipole is instrumental in increasing both the conductivity and the dielectric constant of the electrolyte material. A higher dielectric constant is crucial as it denotes the material’s ability to store electrical energy effectively, paving the way for more efficient battery designs.
Through rigorous experimentation, the researchers discovered that not only do helical structures enhance conductivity, but longer helical forms correlate positively with increased ionic mobility. This correlation implies that manufacturers can potentially tailor the length of the peptide polymers during production, allowing for greater design flexibility in achieving desired conductivity levels. The implications of this finding are vast, as it opens new avenues for optimizing battery performance in real-world applications.
Moreover, the study reveals that the stability of helical polymers outperforms conventional polymers, which often adopt random coil configurations. The robustness of the helical structure minimizes risks associated with thermal degradation under extreme operational conditions. As these polymers maintain their integrity for extended periods, they represent a long-lasting alternative in energy storage systems, thus enhancing overall performance.
In addition to their superior physical properties, helical peptide polymers present a promising solution to the environmental issues associated with traditional batteries. Upon reaching the end of their functional life, these peptide-based materials can be enzymatically or chemically degraded back into their individual monomer units. This degradation process not only reduces waste but also enables the recycling of materials for future use. Such an environmentally friendly approach addresses the pressing need for sustainable practices in energy technology and aligns with global initiatives to minimize ecological footprints.
As the research published in *Nature Materials* demonstrates, the potential of helical peptide structures in solid-state electrolytes marks a significant advancement in the quest for efficient energy solutions. By transforming the basic properties of conventional materials through innovative structural design, scientists are on the brink of a new era in battery technology. The insights drawn from this work pave the way for the development of next-generation solid-state batteries that are not only safe and efficient but also environmentally responsible. Further studies will continue to explore these materials, potentially leading to breakthroughs that can redefine energy storage systems as we know them.

Leave a Reply