In the quest for cleaner and more efficient chemical production, scientists at Lawrence Livermore National Laboratory (LLNL) have made a significant breakthrough by developing a new electrochemical method that promises to transform the industry. With a focus on utilizing thin film nickel anodes, researchers have devised a process that overcomes the limitations of traditional methods, paving the way for a more sustainable future in chemical manufacturing. This innovative approach not only enhances the efficiency of chemical reactions but also reduces their environmental impact, aligning perfectly with global sustainability goals.
The application of thin films in this research is particularly noteworthy. Thin films provide a level surface that eliminates the complexities associated with irregular surfaces that often characterize traditional catalysts. According to LLNL postdoctoral researcher Aditya Prajapati, the use of thin films allows researchers to isolate and examine the intrinsic properties of catalyst materials without the confounding variables that arise from variations in structure. This streamlined approach facilitates a more comprehensive understanding of the electrochemical processes involved, thus empowering the team to refine and enhance reaction efficiencies effectively.
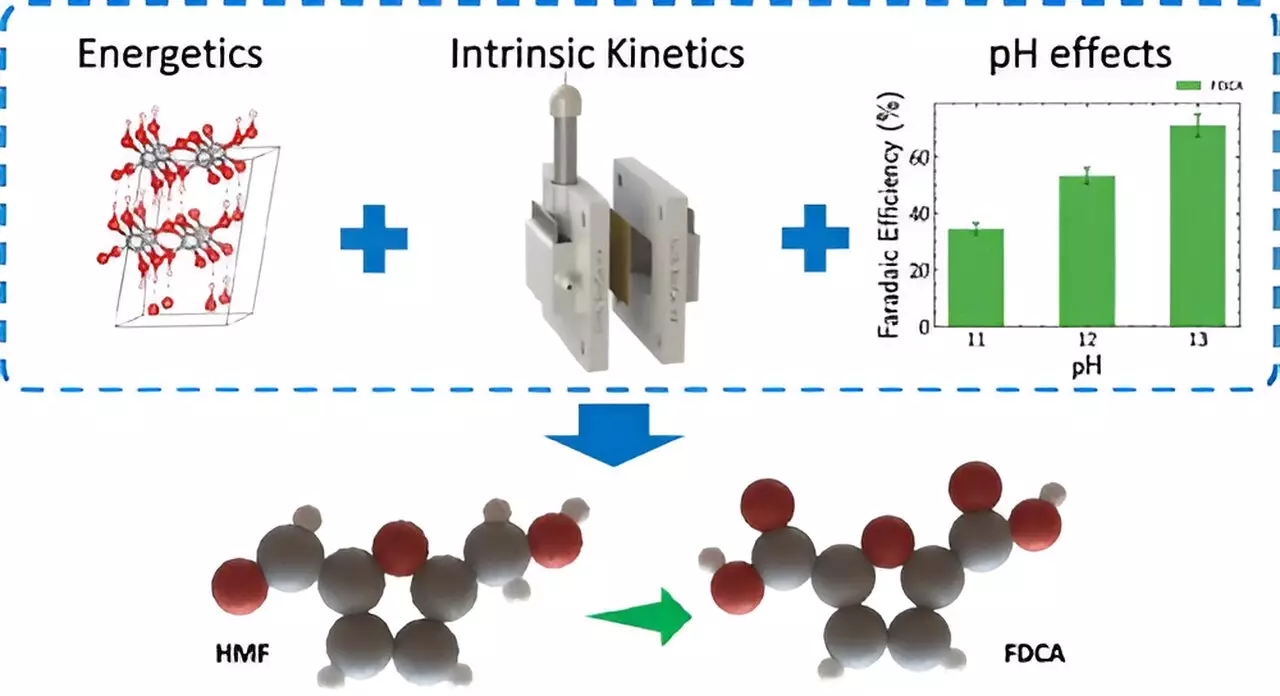
One of the most pressing challenges in contemporary electrolyzer technology is the oxygen evolution reaction, which tends to drain energy efficiency. In a groundbreaking development, LLNL scientists have replaced this oxygen evolution with biomass oxidation, which drastically cuts energy consumption—by over 50%. This pivot from conventional methods highlights the feasibility of using biomass as a renewable resource, such as converting 5-Hydroxymethylfurfural (HMF)—a biobased compound—into 2,5-Furandicarboxylic acid (FDCA), a precursor for renewable plastics like polyethylene furanoate (PEF). These advancements illustrate a promising step towards achieving sustainability in chemical production.
Critically, this innovative electrochemical process presents a viable alternative to traditional methods that often require high temperatures and generate toxic waste products. Not only does the new method offer a cleaner production pathway, but it also contributes to minimizing reliance on petroleum-based products, a significant concern in the fight against climate change. As highlighted by researcher Nitish Govindarajan, the method represents a substantial leap forward in terms of energy efficiency and environmental sustainability.
The potential for achieving a zero-carbon footprint further enhances the promise of this approach when combined with renewable energy sources. This comprehensive consideration of energy inputs and outputs embodies a new paradigm in chemical engineering, where sustainability and innovation go hand in hand.
The collaborative nature of this research has brought together experts from various fields, including contributions from the Université de Montréal and the University of Bonn. Such interdisciplinary collaboration not only enriches the findings but also fosters a community dedicated to driving forward the initiative of sustainable chemistry.
As the scientific community continues to explore these avenues, the implications for industry are profound. The integration of such electrochemical techniques in large-scale chemical manufacturing processes could serve as a significant catalyst for change, demonstrating that sustainability is not just a goal but an achievable reality in the world of chemical production.

Leave a Reply