Z-alkenes are no ordinary players in the world of organic compounds; they represent a unique structural arrangement characterized by a double bond between carbon atoms, with substituents positioned on the same side. This configuration not only grants them interesting chemical properties but also makes them important components in various biological and industrial applications. Their production, however, is not as straightforward as one might assume. Traditional strategies in organic synthesis often fall short when it comes to generating Z-alkenes efficiently. Instead, the innovative method of photoisomerization emerges as a beacon of promise, illustrating the dynamic potential of light in transforming molecular structures.
Photoisomerization refers to the phenomenon where light energy induces a transformation in the arrangement of atoms within a molecule, effectively converting one isomer into another. This technique offers intriguing advantages, particularly in the realm of converting E-alkenes, the more stable isomer, to their Z counterparts. As scientists relentlessly probe the depths of this method, they unveil an array of possibilities across various fields such as synthetic organic chemistry, materials science, and pharmaceuticals. The process not only simplifies the synthetic pathways but also enhances yields and reduces waste, aligning beautifully with modern demands for environmentally friendly approaches.
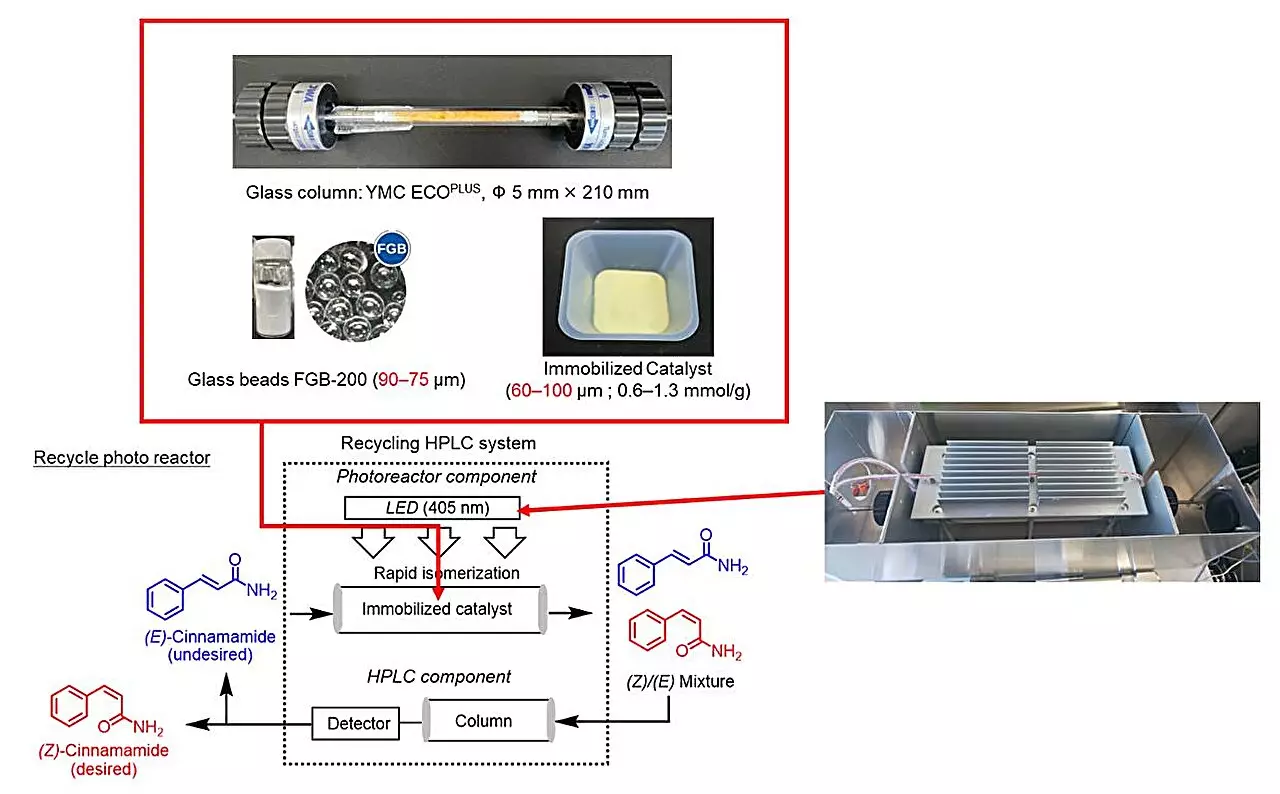
In their relentless pursuit of efficient photoisomerization methods, researchers, particularly a team led by Professor Hideyo Takahashi at Tokyo University of Science, have pioneered a groundbreaking approach involving a recycling photoreactor. This novel system merges principles from traditional organic chemistry with cutting-edge technologies, including high-performance liquid chromatography (HPLC). By employing a continuous closed-loop process, the researchers have devised a method that cleverly couples the photoconversion of E-cinnamamides into Z-cinnamamides while simultaneously recycling the components involved. This addresses the common stumbling block of waste—enabling chemists to enhance productivity while championing ecological sustainability.
The transition from E-cinnamamides to Z-cinnamamides hinges on the use of photosensitizers—compounds capable of absorbing light and facilitating the necessary chemical transformations. The team’s meticulous screening process identified thioxanthone as the most effective candidate. What’s particularly remarkable is the strategy of immobilizing thioxanthone on modified silica gel. This leap not only curbed the leakage of photosensitizers but also significantly bolstered catalytic efficiency, an outcome contrary to conventional expectations that solid-phase reactions typically lag behind their liquid-phase counterparts. By cleverly integrating functional groups into this approach, the researchers have opened avenues for enhancing reaction rates, pushing the boundaries of what’s possible in organic synthesis.
The implications of this innovative recycling photoreactor extend far beyond merely generating Z-alkenes. The research team has set the stage for a transformative paradigm in organic synthesis, one that boldly intertwines efficiency with sustainability. Emphasizing the closed-loop nature of their method, they advocate for this approach as not just technically sound but also environmentally responsible. With the pharmaceutical industry facing increasing scrutiny regarding its ecological footprint, adapting such methods could yield a plethora of Z-alkenes, crucial for drug development, with dramatically reduced environmental repercussions.
The work of Professor Takahashi and his colleagues encapsulates the essence of modern scientific inquiry—melding tradition with innovation to create pathways that honor both efficiency and ecological integrity. As they continue to refine their methods, the journey towards a more sustainable future in organic chemistry is not just a possibility; it’s an imperative. By embracing these forward-thinking practices, researchers can pave the way for advancements that consider not only the complexities of molecular design but also the broader context of their environmental impact. The future of chemistry lies in balancing these two essential dimensions—where innovation breathes new life into age-old practices, creating sustainable solutions that resonate within the scientific community and beyond.

Leave a Reply