In the realm of medical science, innovation often takes strange and unexpected turns. One such turn is the burgeoning field of photopharmacology, a discipline that explores how light can be harnessed to activate and control drug delivery in targeted areas of the body. Radical advancements in this domain promise to revolutionize how we approach pain management and many other medical applications. Central to this innovation is the concept of employing light-activated molecular switches, which are integrated into drug compounds to control their activation solely in the presence of specific light wavelengths.
One of the most promising applications of photopharmacology lies with the development of novel derivatives of existing medications. The team at the Institute for Bioengineering of Catalonia (IBEC) has taken significant steps by creating photoswitchable analogs of carbamazepine, a well-established anti-epileptic medication. By modifying the drug’s chemical structure to include an azobenzene-based switch, researchers are enabling a new level of localized treatment that could lead to unprecedented efficiency in analgesic delivery.
Targeted Approaches to Neuropathic Pain Relief
Neuropathic pain—often characterized by its long-lasting, debilitating nature—presents a significant challenge in medical treatment. Common causes include injuries to the somatosensory system and conditions like diabetic neuropathy and lumbar radiculopathy, which can result in uncomfortable sensations that persist long after the initial injury has healed. Conventional treatments often involve opioids or non-steroidal anti-inflammatory drugs (NSAIDs), both of which carry considerable risks of side effects and addiction. Consequently, researchers are understandably excited about the potential ramifications of a localized, non-invasive treatment model that photopharmacology offers.
The newly engineered compounds, specifically carbazopine-1 and carbadiazocine, have shown promising results in preclinical trials. These compounds activate upon exposure to amber light wavelengths, which can penetrate tissues and bones efficiently. Such capacity allows for precise control over how and when the drug effects take place, thus minimizing the systemic side effects commonly associated with traditional analgesics. This level of control could significantly improve patient outcomes, freeing many from the vicious cycle of reliance on more dangerous pain relief options.
Real-time Control and Reversible Effects
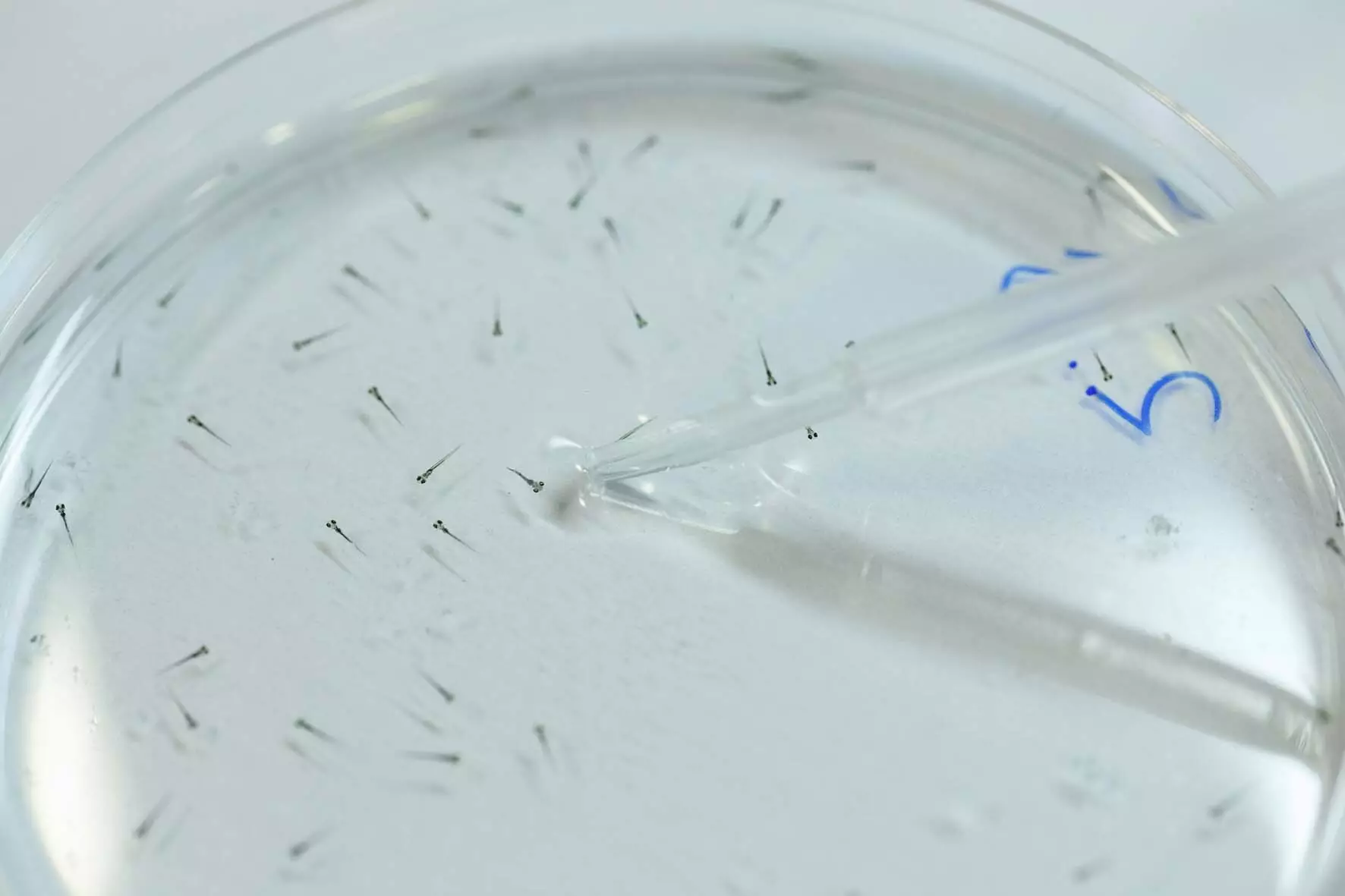
A particularly captivating aspect of this research lies in the compounds’ reversible effects, effectively allowing clinicians and patients to control pain responses in real-time. In experiments involving zebrafish larvae, for instance, researchers observed that light exposure could not only speed up movement but also slow it down again with the change in wavelength. Such fine-tuned manipulation of the nervous system could lead to exciting new treatment modalities that can adapt to a patient’s immediate needs, representing a seismic shift away from one-size-fits-all approaches.
Luisa Camerin, an eminent researcher at IBEC, highlighted the effects in nonsedative terms as patients can experience relief without the dysfunction typically associated with heavier drugs. Carbadiazocine, in particular, has demonstrated analgesic properties without inducing anesthesia, sedation, or toxicity in laboratory rat models. This ensures a safer profile, making ways for a more compelling argument for their clinical integration.
The Broader Implications for Medicine
As the research team at IBEC progresses towards the next phase, involving infrared light that penetrates tissues even deeper, the implications of these advancements extend far beyond pain management. The ability to deliver medications targeted by light could essentially reshape how treatments for various diseases are orchestrated. From cancer therapies that activate drug delivery to controlling inflammation, this methodology lays the groundwork for a multitude of targeted therapies that could reduce general body-wide drug exposure.
In a system often reliant on generalized medications, the specificity and adaptiveness present in light-activated therapies should not only amplify effectiveness but also minimize unwanted side effects. As we edge closer to unleashing the full potential of photopharmacology, one can’t help but speculate about the future of medicine, where healing is no longer a blunt force, but rather a precise and elegant orchestration of light-powered interventions. The prospect of portable light sources such as lasers or LEDs ushering in this change signals a new dawn in medical treatment strategies, one that is innovative, effective, and undeniably exciting.

Leave a Reply