The proliferation of micropollutants—substances such as pesticides, pharmaceuticals, and industrial chemicals—poses an increasing threat to our water supplies and ecosystems. These tiny but potent contaminants resist traditional wastewater treatment methods, leading to a pressing need for innovative technologies capable of addressing this environmental dilemma. Traditional methods often prove inefficient at removing these small molecules, demanding a paradigm shift in our approach to environmental remediation. The search for effective solutions requires not only understanding the contaminants themselves but also harnessing advanced technologies that can effectively counter their destructive impacts on the environment.
Introducing Photocatalysis
Among the numerous potential solutions, photocatalysis has emerged as a formidable technique, leveraging semiconductor materials to degrade harmful substances through a process activated by light. At the heart of this method is titanium dioxide (TiO2), a naturally abundant and cost-effective semiconductor. By virtue of its ability to absorb light and generate electron-hole pairs, TiO2 can catalyze redox reactions that break down various pollutants. However, the inherent challenge lies in enhancing the efficiency of this process, particularly by improving the adsorption capabilities of TiO2 to increase the interaction between the catalyst and micropollutants.
Gold Particles: A Game Changer
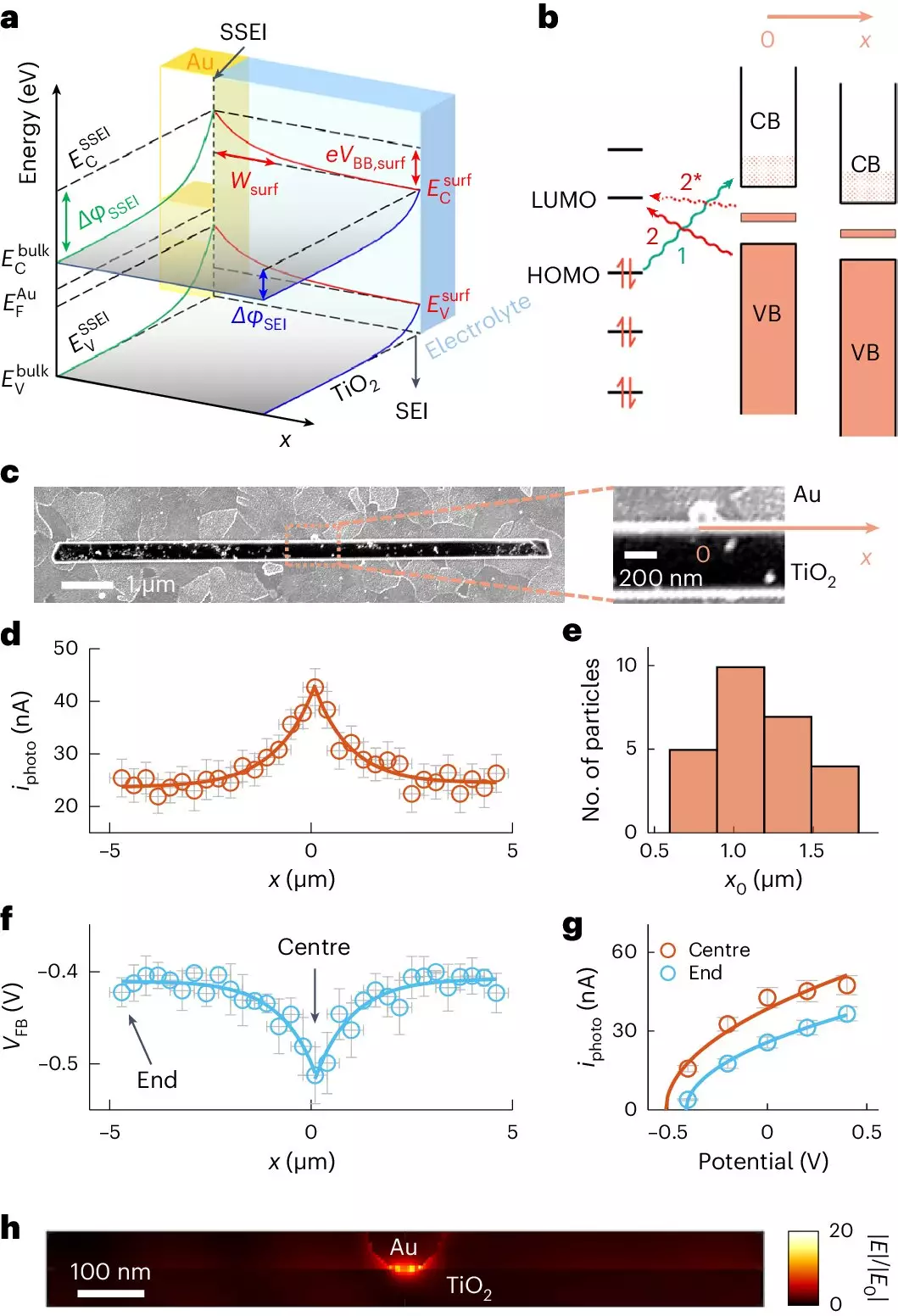
Recent research from a Cornell University team led by Peng Chen has spotlighted the transformative potential of integrating gold nanoparticles into TiO2 photocatalysis. Gold, a noble metal, has desirable electronic properties that can significantly improve the catalytic activity of TiO2. The team’s exploration of this synergy is not merely a theoretical exercise; they employed a cutting-edge imaging technique, adCOMPEITS (Adsorption-based COMPetition Enabled Imaging Technique with Super-resolution), to observe the adsorption dynamics on TiO2. This innovative approach allows for a granular view of how pollutants interact with the catalyst at previously unattainable resolutions.
The findings suggest that the presence of gold enhances adsorption capabilities significantly—even at distances of up to a micrometer from the nanoparticle itself. This revelation challenges conventional wisdom regarding the localized effects of catalysts, demonstrating that these enhancements can reach far beyond the immediate vicinity of the gold particle. The impact of this “long-range adsorption enhancement” appears to hinge on changes in electronic properties at the TiO2 surface, a development Chen describes as “surface band bending.”
Implications for Photocatalysis Efficiency
The implications of this research are profound. Improving the efficiency of photocatalysis not only addresses the issue of micropollutants in wastewater but opens avenues for broader applications across various domains. The capacity to enhance adsorption with minimal amounts of gold nanoparticles means that the overall cost and resource requirements for photocatalytic systems can be drastically reduced. This is crucial in scaling the technology for real-world applications, particularly in developing countries where financial constraints can limit environmental management efforts.
Moreover, the findings cast a spotlight on opportunities that extend beyond wastewater treatment. The principles derived from this research could inform advancements in fields such as sensing technologies and energy conversion mechanisms, including dye-sensitized solar cells. As societies grapple with energy transitions toward greener alternatives, the principles outlined in Chen’s research could pave the way for eco-friendly technologies that seamlessly integrate waste management and energy solutions.
Broader Applications and Future Directions
The versatility of the technology holds promise for various sectors, from agriculture to renewable energy. Enhanced photocatalytic systems can serve not only in treating contaminated water but also in creating sustainable agricultural practices through the reduction of pesticide residues. Additionally, the rapid advancement of nanotechnology means that future research will likely unveil even more efficient co-catalysts, enabling more potent combinations for environmental restoration.
Ultimately, the collaborative work conducted by interdisciplinary teams highlights the critical interplay between chemistry, engineering, and environmental science in tackling pressing global challenges. By pushing the boundaries of our scientific understanding and embracing innovative methodologies, we can forge pathways toward cleaner ecosystems and a healthier planet. The findings from this Cornell study stand as a testament to the remarkable potential of modern science to deliver impactful solutions for some of humanity’s most pressing concerns.

Leave a Reply