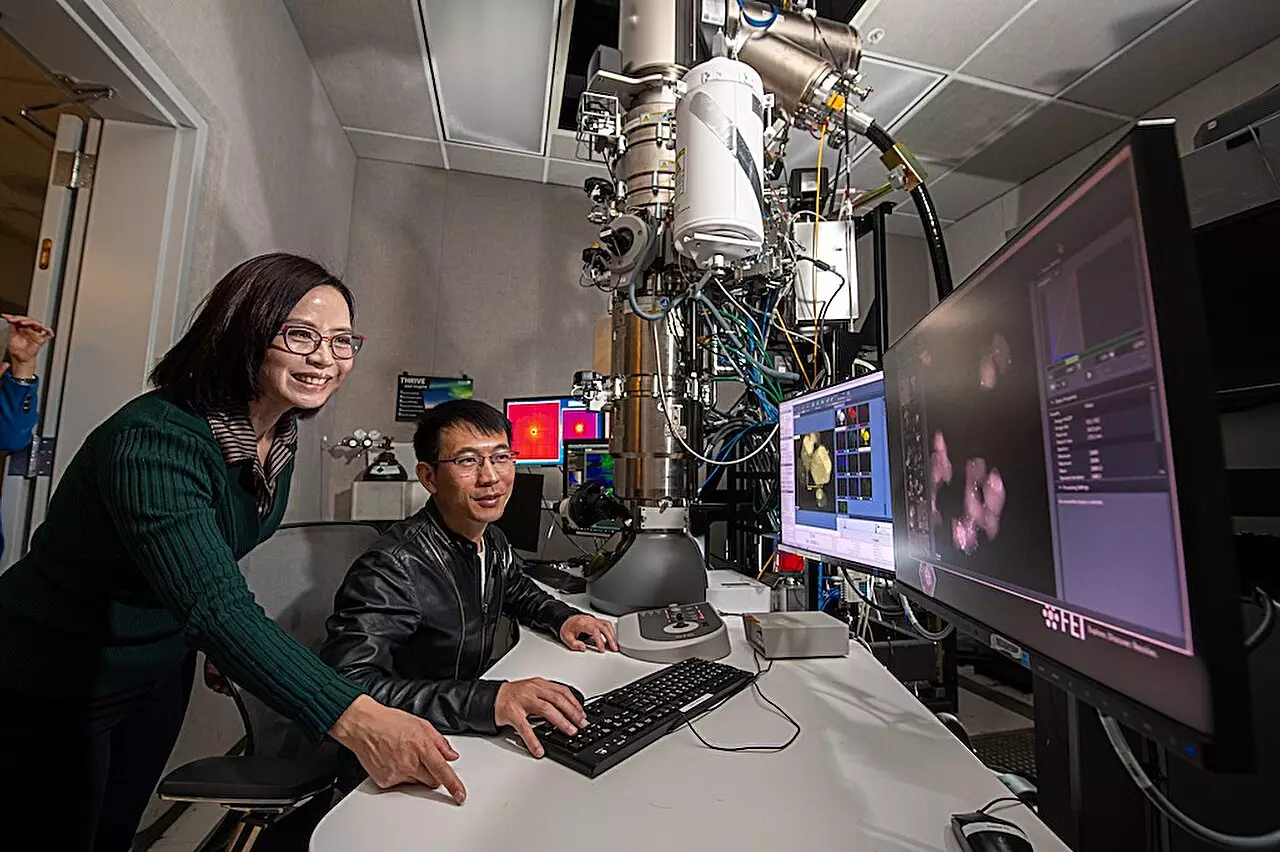
Innovative developments in research often stem from the need to understand complex phenomena that are fundamental to our technological ecosystem. A pioneering team from the Lawrence Berkeley National Laboratory (Berkeley Lab) has made a monumental breakthrough in the study of electrochemical processes, crucial for applications ranging from batteries to fuel cells. The research, led by Haimei Zheng, unveils an ingenious method to observe electrochemical reactions at the atomic scale with unprecedented resolution. This novel approach not only enhances our understanding of catalysts but also holds transformative potential for a multitude of energy-related fields.
Electrochemical reactions represent the foundational mechanisms driving many of today’s essential technologies, including solar fuel generation and electrolysis. Despite their critical importance, the intricate details of these reactions, particularly the interactions occurring at the atomic level, have remained largely enigmatic. The introduction of a polymer liquid cell (PLC) equips scientists with the ability to visualize these interactions in real time, offering a glimpse into the molecular dance occurring during electrochemical reactions.
Understanding the Polymer Liquid Cell
The PLC is a remarkable creation designed to facilitate an in-depth analysis of electrochemical reactions. Essentially, it is a tightly sealed chamber capable of hosting all necessary components for a reaction while also being observably paired with transmission electron microscopy (TEM). The most groundbreaking feature of this cell is its ability to freeze the reaction at various time points. This tactical pause allows researchers to capture minute details of the reaction’s progress and crystalize our understanding of the catalysis process, thus paving the way for technological advancements in catalytic design.
According to Zheng, the PLC allows for real-time observation of solid-liquid interfaces, a complex aspect of electrochemical reactions that has historically eluded scrutiny. The findings, discussed in a recent paper in *Nature*, emphasize the importance of observing how catalyst atoms shift and evolve under reaction conditions. By witnessing these transformations, scientists hope to glean valuable insights into both the functionality and degradation of catalysts.
Diving Into Copper Catalysts
Among the various applications of the PLC, the focused investigation on copper catalysts has been particularly illuminating. The copper system studied is a subject of intense research due to its capacity to convert atmospheric carbon dioxide into valuable hydrocarbons, such as methanol and acetone. However, accomplishing this efficiently while minimizing off-target products remains a significant challenge.
Utilizing the advanced capabilities of the Berkeley Lab’s National Center for Electron Microscopy, researchers probed the solid-liquid interface critical to the reaction. They observed how copper atoms manifested and intermixed with other elements to create a metastable “amorphous interphase”—a state indicative of a previously misunderstood dynamic interaction. The observation of this elusive phase pushes the boundaries of existing knowledge on solid-liquid interfaces, positioning the research as a potential game-changer in catalyst engineering.
Implications for Future Research
The discovery of the amorphous interphase is not merely a footnote in the field; it prompts a complete re-evaluation of strategies related to the design and optimization of electrochemical catalysts. While traditional approaches have prioritized static models based on initial surface structures, the new insights from the PLC suggest that dynamic conditions during reactions significantly influence catalyst performance.
Researchers like Qiubo Zhang and Zhigang Song underscore the potential advantages of leveraging the amorphous interphase dynamics for developing more selective catalysts. By interrogating the changing structure of this interphase, scientists could devise methods to enhance reaction efficiencies and extend the durability of catalysts—a crucial issue given the tendency of catalysts to degrade over time.
Looking Ahead: A New Dawn in Electrochemical Technology
The excitement surrounding the PLC technology is palpable within the scientific community, with researchers eagerly anticipating its application to other electrochemical materials, including lithium and zinc catalysts. The technology promises not only to deepen our understanding of existing catalysts but also to motivate the development of innovative materials designed for specific outcomes.
The advances achieved by the Berkeley Lab team exemplify the profound impact that elucidating atomic-level processes can have on technology. By refining our understanding of catalysts and their behavior, this research paves the way for a more sustainable and efficient future in energy production and utilization. The responsibility now lies on the academic and industrial researchers alike to harness these insights, guiding us towards an era of cleaner energy solutions and advanced catalytic systems.

Leave a Reply