A new frontier in sustainable energy is emerging through innovative research at the University of Michigan, where scientists are pioneering a method of reusing carbon dioxide (CO2) to produce essential hydrocarbons. This process is key to creating sustainable fuels, as carbon atoms are chained together in a groundbreaking artificial photosynthesis system that significantly outperforms existing methodologies. By efficiently converting CO2 into ethylene—a primary compound used in plastic production—this system offers a promising pathway to mitigate carbon emissions while fulfilling material needs in a world increasingly dependent on plastics.
The research led by Zetian Mi, a professor in electrical and computer engineering, highlights impressive performance metrics that set this new system apart. With an efficiency and stability of operation five to six times superior to traditional solar energy-driven CO2 reduction methods, the implications are profound. Ethylene, despite being the most produced organic compound globally, faces steep environmental costs when produced conventionally through fossil fuels. The high temperatures and pressures associated with traditional ethylene production contribute significantly to global CO2 emissions, making this alternative process not only desirable but vital for achieving sustainability goals.
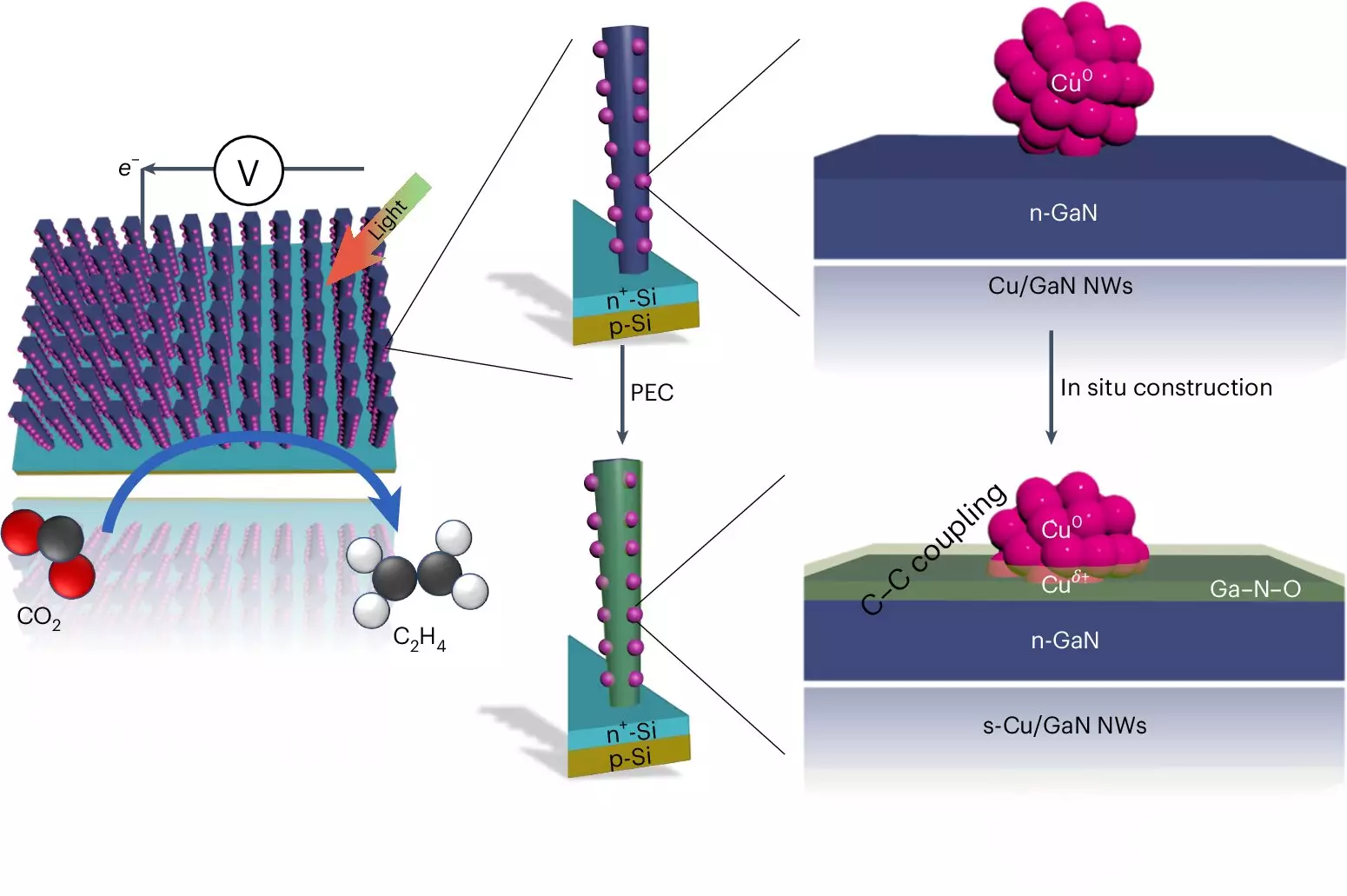
The Michigan team’s innovation focuses specifically on overcoming the traditional barriers of high energy requirements and carbon emission outputs. By inventively using sunlight as an energy source, the system absorbs light through specially created nanowires made from gallium nitride, alongside a silicon base. This technological leap allows for better energy conversion and utilization compared to past methods, which often struggled with efficiency and longevity.
At the heart of this artificial photosynthesis system lies a sophisticated reaction mechanism involving copper clusters that act as a catalyst. These clusters facilitate a transformative chemical process whereby carbon dioxide and water are combined to yield ethylene. Interestingly, this reaction occurs when gallium nitride nanowires, submerged in CO2-enriched water, are exposed to sufficient sunlight. This system splits water molecules near the nanowire surfaces to release hydrogen—an essential ingredient that fuels the ethylene production—while simultaneously producing oxygen, which contributes to the sustainability of the process.
In essence, the copper clusters capture hydrogen and bond it with carbon atoms extracted from CO2. The process culminates at the interface of the copper and gallium nitride oxide, showcasing a remarkable interaction: oxygen atoms are stripped from the carbon dioxide and replaced by hydrogen atoms derived from water. This transition underlines a fundamental principle in chemistry—the transformation of reactants into useful products.
Astoundingly, the systems developed by the University of Michigan not only match but exceed their competitors in longevity and efficiency. While alternative catalysts based on silver and copper displayed commendable efficiency, their operational lifespan was severely limited—lasting only a few hours before degradation. In stark contrast, the Michigan team’s device functioned continuously for 116 hours without any drop in performance, and even accumulated operational hours nearing 3,000 in previous tests. This characteristic is largely attributed to the synergistic relationship between the gallium nitride and the ongoing water-splitting reactions, as the presence of oxygen enhances the catalyst’s effectiveness and promotes self-healing characteristics.
The sustained production of ethylene is merely the starting point for future explorations. Researchers envision broadening the scope of this technology to produce more complex hydrocarbons and fuels such as propanol and other multi-carbon compounds. These advancements could revolutionize transportation technologies, aligning them with sustainability goals and reducing reliance on fossil fuels.
As the team continues to refine their technology, the long-term implications become clearer: the ability to create liquid fuels that align with existing technologies could profoundly reshape sectors hindered by carbon emissions and environmental concerns. The continuation of this groundbreaking research promises not just a reduction in atmospheric CO2 but a comprehensive strategy for integrating sustainability into our everyday material needs.
The research emerging from the University of Michigan symbolizes a critical step towards harnessing carbon emissions for productive purposes. This novel technique promises to bridge the gap between sustainable energy solutions and real-world applications, offering a potent mechanism to convert greenhouse gases into valuable hydrocarbons. As the journey towards creating a greener future unfolds, advancements in artificial photosynthesis stand poised to make a tangible difference in addressing both environmental challenges and global material demands.

Leave a Reply