In an impressive advancement for sustainable chemistry, researchers have successfully transformed thiols and disulfides into sulfonyl fluorides using the novel combination of SHC5 and potassium fluoride (KF). This innovative method presents a remarkable leap forward in the realm of click chemistry, which is celebrated for its high efficiency, selectivity, and minimal environmental footprint. Published in ACS Sustainable Chemistry & Engineering, the findings promise to reshape synthetic protocols in both laboratory and industrial contexts.
Click chemistry’s paradigm revolves around rapid, reliable coupling of molecules, making it crucial for a plethora of applications—from pharmaceutical synthesis to materials science. Yet, the synthesis of sulfonyl fluorides, essential participants in sulfur-fluorine exchange (SuFEx) reactions, had presented significant challenges previously. Traditional methods relied on hazardous materials like SO2F2 and KHF2, which posed significant safety and toxicity risks for chemists.
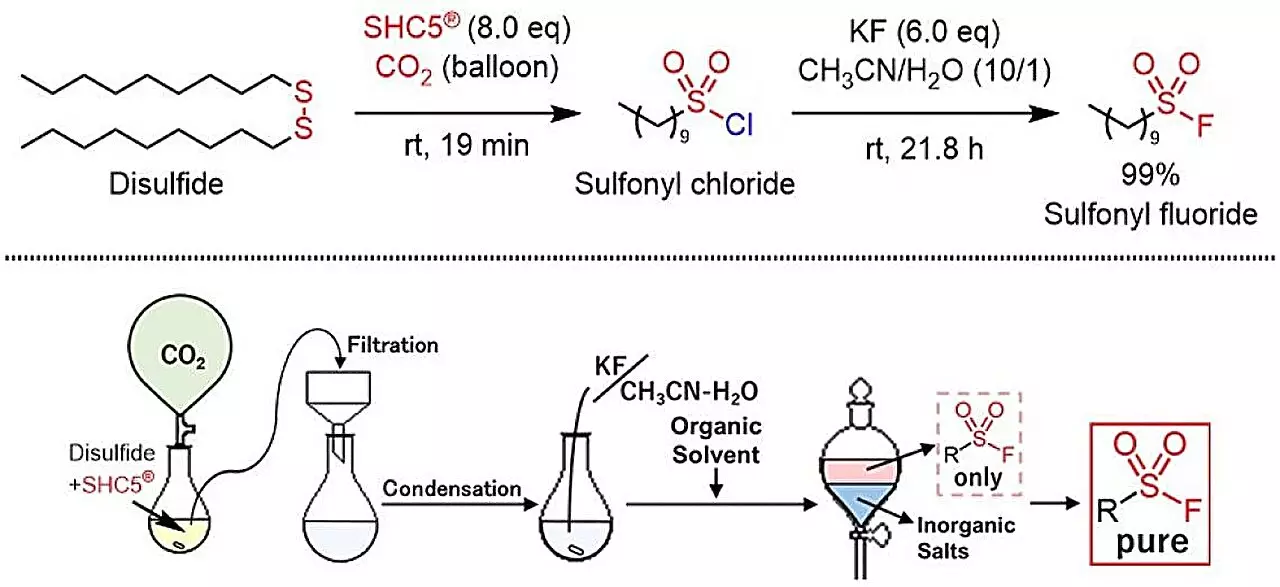
The research team behind this study sought a greener alternative and has made a monumental breakthrough. By utilizing SHC5 in combination with KF, the researchers demonstrated that sulfonyl fluorides can now be synthesized safely and efficiently. This pathway yields benign by-products—sodium chloride and potassium chloride—thereby significantly reducing the ecological impact compared to existing methods.
The scalability of this new method positions it as a game-changer for manufacturers and chemists alike. The operational simplicity of the chemical process allows for cost-effective production while ensuring safety. This is particularly vital when looking to expand on the industrial application of sulfonyl fluorides, which are widely sought after in the development of pharmaceuticals and diverse organic compounds.
Notably, the implications of this research extend beyond mere chemical advancements; they resonate with global efforts focused on Sustainability Development Goals (SDGs). In their publication, lead researchers Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem emphasize the importance of eco-conscious methodologies in organic synthesis. The importance of evaluating the environmental impact of chemical reactions has never been more critical, especially as industries face increasing scrutiny for their ecological footprints.
In promoting sustainable practices, this research not only emphasizes the practical utility of sulfonyl fluorides, but also highlights the pressing need for the chemistry sector to evolve. Safer, greener synthetic methods stand to benefit not only the chemical industry but align tightly with broader social and environmental objectives.
The development of a green synthetic method for sulfonyl fluorides signifies a remarkable milestone in the field of chemistry. As the sector moves toward a more sustainable future, innovations like those described could redefine not just the synthesis of valuable compounds, but also set a precedent for how future methods are developed and implemented. Embracing these advancements reflects a proactive step toward balancing chemical innovation with the urgent need for sustainability, paving the way for a more responsible approach to scientific discovery and industrial practices.

Leave a Reply