Recent advancements in biochemistry have unveiled critical insights into the enzymatic processes that facilitate cancer growth, particularly involving serine hydroxymethyltransferase (SHMT). A research team from the Department of Energy’s Oak Ridge National Laboratory (ORNL) employed neutron experiments to delve into the vital functions of this enzyme, a pivotal component in the metabolic pathways regulating cell division. Their findings hold significant promise for enhancing drug design strategies aimed at combating aggressive cancers, ushering in hope for more effective treatments.
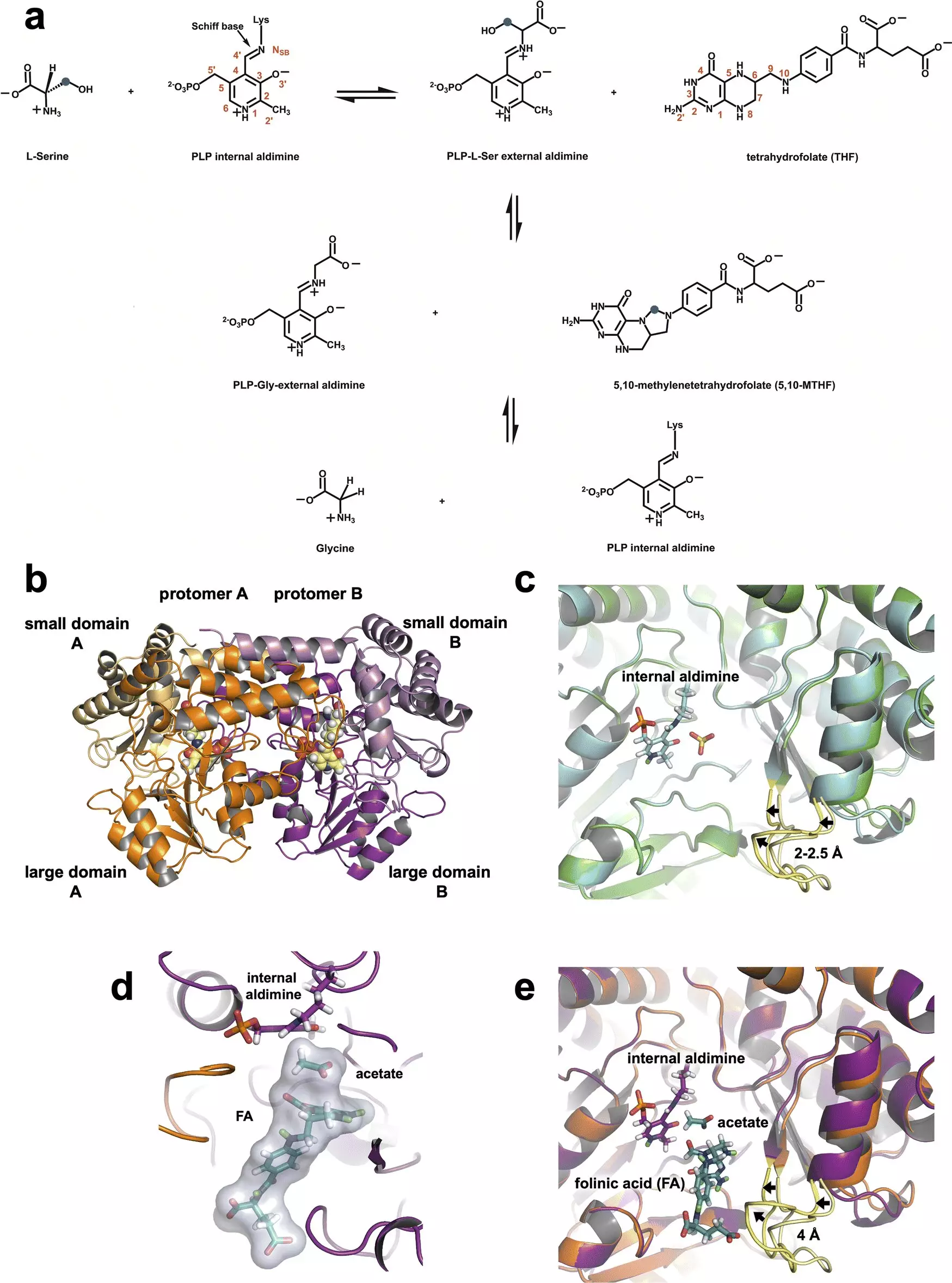
SHMT plays a crucial role in one-carbon metabolism, a pathway that enables cells to convert serine into glycine while transferring the necessary carbon atom to tetrahydrofolate. This biochemical exchange is essential for synthesizing nucleic acids, such as DNA and RNA, which are fundamental for cell proliferation. However, in the presence of cancer, the functionality of SHMT becomes aberrant as cancer cells manipulate metabolic processes to fuel their relentless reproduction. Research highlights that cancerous cells often exhibit an overproduction of SHMT, transforming this vital enzyme into a double-edged sword that accelerates their growth.
The innovative use of neutron diffraction at facilities like the Spallation Neutron Source (SNS) and the High Flux Isotope Reactor (HFIR) allowed researchers to probe the atomic-level structures and functions of SHMT with unprecedented clarity. Neutron scattering is particularly adept at revealing the positions of light elements, notably hydrogen, which are crucial for understanding enzymatic functions. The research team led by ORNL’s Victoria Drago successfully demonstrated how hydrogen interactions define SHMT’s catalytic mechanism, a topic that has long been shrouded in ambiguity.
By elucidating the roles that various amino acids play in the active site of SHMT, the team uncovered that a single glutamate residue is essential for the enzyme’s catalytic function. This residue effectively carries protons, allowing SHMT to function both as an acid and a base, thus enabling the critical transfer of carbon atoms within the cell’s metabolic machinery.
The precise understanding gained from this study can inform the design of SHMT inhibitors—compounds that can potentially stall the enzyme’s function within the cancerous metabolic pathway. By identifying how SHMT engages with its substrates, the research team gains crucial insights into how to effectively block its activity with strategic small-molecule inhibitors. This could represent a groundbreaking approach in the development of combination therapies that target the metabolic vulnerabilities of cancer cells.
Current cancer therapies often lead to severe side effects because they indiscriminately attack both cancerous and healthy cells. By targeting SHMT specifically, there is the potential to enhance treatment efficacy while minimizing collateral damage to normal tissues. This distinction is critical, setting a foundation for the more precise application of targeted therapies in oncology.
The research illustrates a burgeoning area of interest in the interplay between neutron-scattering technologies and medicinal chemistry. As William Nelson, director of the Sidney Kimmel Comprehensive Cancer Center, noted, the coupling of discoveries in enzymatic structures with advanced technologies could revolutionize the way drugs are developed and personalized in treating cancers. The promise of AI-driven approaches to sequence cancerous genes and predict protein structures can lead to the rapid design of specific inhibitors, potentially in a timespan of mere hours.
However, while the prospects are exciting, it is essential to temper expectations. The complexity of cancer biology, characterized by its adaptability and stealthy metabolic hijacking, poses significant challenges. Unlike infectious diseases, where mechanisms of action and target proteins can be more straightforward, cancer necessitates a more nuanced understanding due to the risk of harming healthy cells along with malignant ones.
The ORNL study on SHMT underscores an essential advance in our understanding of cancer metabolism and the potential for new therapeutic avenues. By unravelling the intricate details of SHMT’s function through experimental techniques such as neutron diffraction, researchers are paving the way for transformative approaches in drug development. These findings resonate with hope that future cancer therapies will be more precise, targeted, and ultimately more effective in combating one of humanity’s most challenging adversaries. The integration of advanced methodologies and computational tools will be indispensable as we move forward in the long journey towards conquering cancer.

Leave a Reply