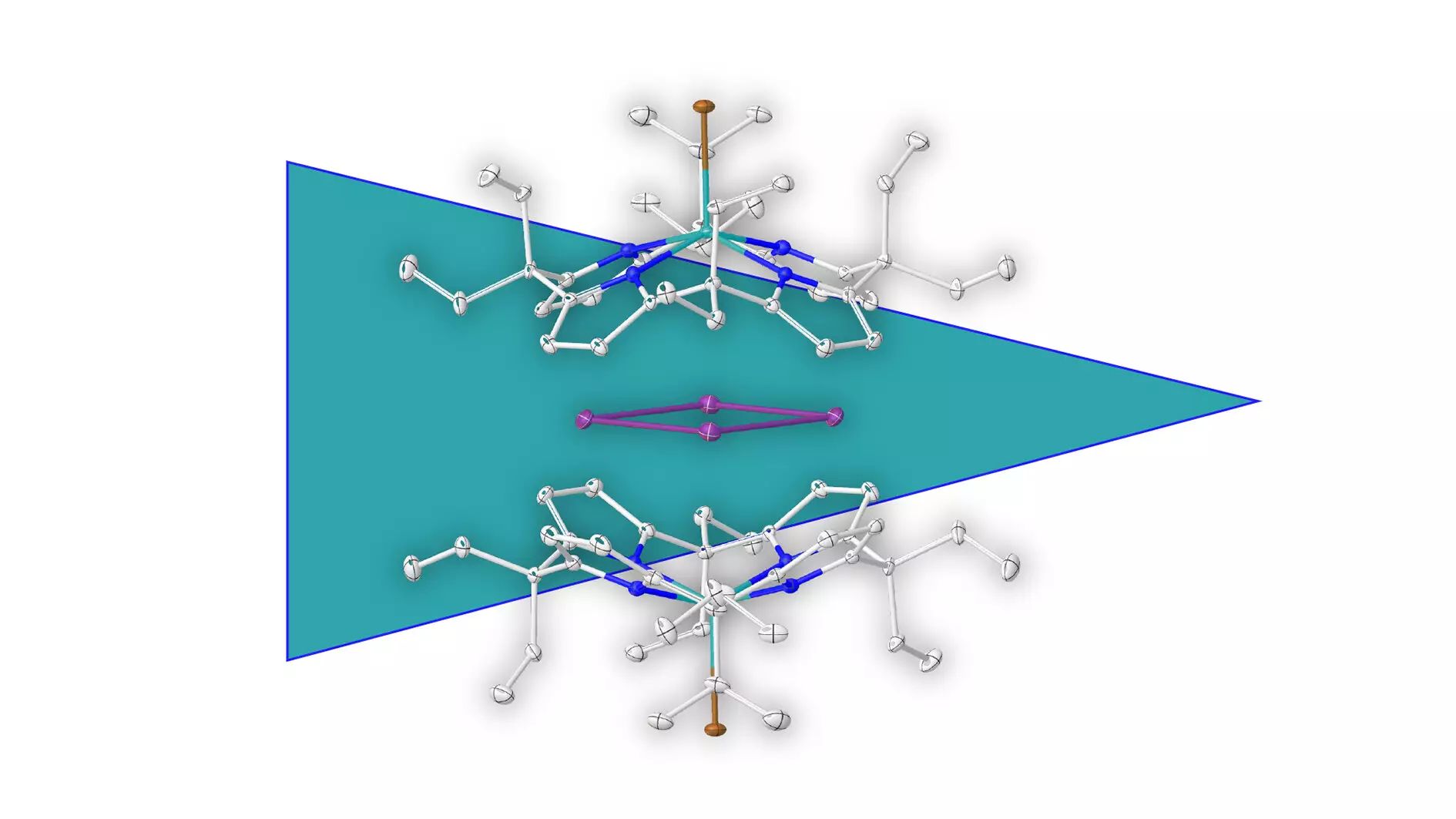
The realm of chemistry is constantly evolving, presenting fresh insights and discoveries that challenge existing paradigms. One of the most exciting recent developments comes from a research team at Heidelberg University, led by Prof. Dr. Lutz Greb, which has unveiled the existence of a metal-based aromatic ring made entirely of bismuth atoms. This groundbreaking discovery not only expands our understanding of aromaticity but also opens up new avenues for exploration in inorganic chemistry.
Aromaticity, traditionally associated with cyclic compounds that exhibit unique stability due to their delocalized pi electrons, has historically been limited to carbon-based structures. The newfound discovery of an aromatic metal ring challenges long-held beliefs within the scientific community. With its composition solely of elemental bismuth, this ring diverges from the established understanding of aromatic systems and suggests that aromatic characteristics can transcend the boundaries of organic compounds.
Surprising Stabilization Techniques
One of the most intriguing aspects of this research is the innovative method employed to stabilize the metal ring. By encapsulating the electron-deficient bismuth ring in a negatively charged molecular shell, the researchers prevented the ring from decomposing. This supramolecular stabilization approach represents a pivotal breakthrough, showcasing the potential for developing new stabilization techniques for positively charged species. This opens up avenues not just for metal aromatic structures but also for a variety of other positively charged molecular cages.
Prof. Greb’s assertion that this technique could be applied more broadly highlights the versatility of the approach. By leveraging molecular interactions that create an environment conducive for stabilization, chemists may discover alternative methods to facilitate the study and utilization of other reactive metal complexes.
Implications for Charge Transport and Beyond
The implications of this work extend far beyond mere academic interest. The discovery of metal-based aromatic compounds holds promise for enhancing our understanding of charge transport mechanisms in metals. As electronic devices become increasingly miniaturized, understanding charge transport on a molecular level is crucial for developing efficient materials. Aromatic metal complexes might offer unique pathways for electron movement, potentially leading to breakthroughs in semiconductors and conductive materials.
Furthermore, the insights gained from this study could inspire new designs for catalysts, sensors, and other materials where metal interactions play a critical role. This new class of aromatic compounds might provide unexpected properties that can be tailored for specific applications, effectively bridging the gap between fundamental research and real-world applications.
A Paradigm Shift in Chemical Perception
Ultimately, Prof. Dr. Lutz Greb and his team’s work represents a paradigm shift, not only in the intelligence of aromaticity but also in how we perceive the capabilities of metal-centric compounds. This discovery diminishes the once-rigid boundaries separating organic and inorganic chemistry, suggesting a more integrated approach to studying molecular structures. As researchers build upon this foundation, we may be witnessing the dawn of a new era in material science—one that is rich with potential and ripe for exploration. The ventures into metal aromaticity could redefine how we think about chemistry itself, steering us toward uncharted territory.

Leave a Reply