The ongoing quest to find effective solutions for carbon dioxide (CO2) emissions—an unavoidable byproduct from diverse sources including energy production and transportation—has led researchers to explore innovative methodologies. Among these, electrochemical reduction stands out as a potential game changer. This process not only aims to mitigate CO2 emissions but also seeks to transform captured carbon into valuable fuels like methanol and ethanol. However, a significant barrier remains the identification of a catalyst that is both efficient and rapid enough for practical applications. Recent advancements from a collaborative research effort involving Brookhaven National Laboratory, Yale University, and the University of North Carolina provide renewed optimism in this field.
A major drawback faced by many current catalysts in CO2 reduction is their energy inefficiency. High-energy inputs are often necessary to facilitate the conversion process, limiting their scalability and economic viability. As highlighted by Brookhaven chemist Gerald Manbeck, the favored catalyst needs to operate effectively with minimal energy consumption. This highlights a crucial balance: achieving high catalytic performance while keeping energy demands low. The pursuit of this balance is imperative for achieving widespread adoption of electrochemical avenues in carbon reduction.
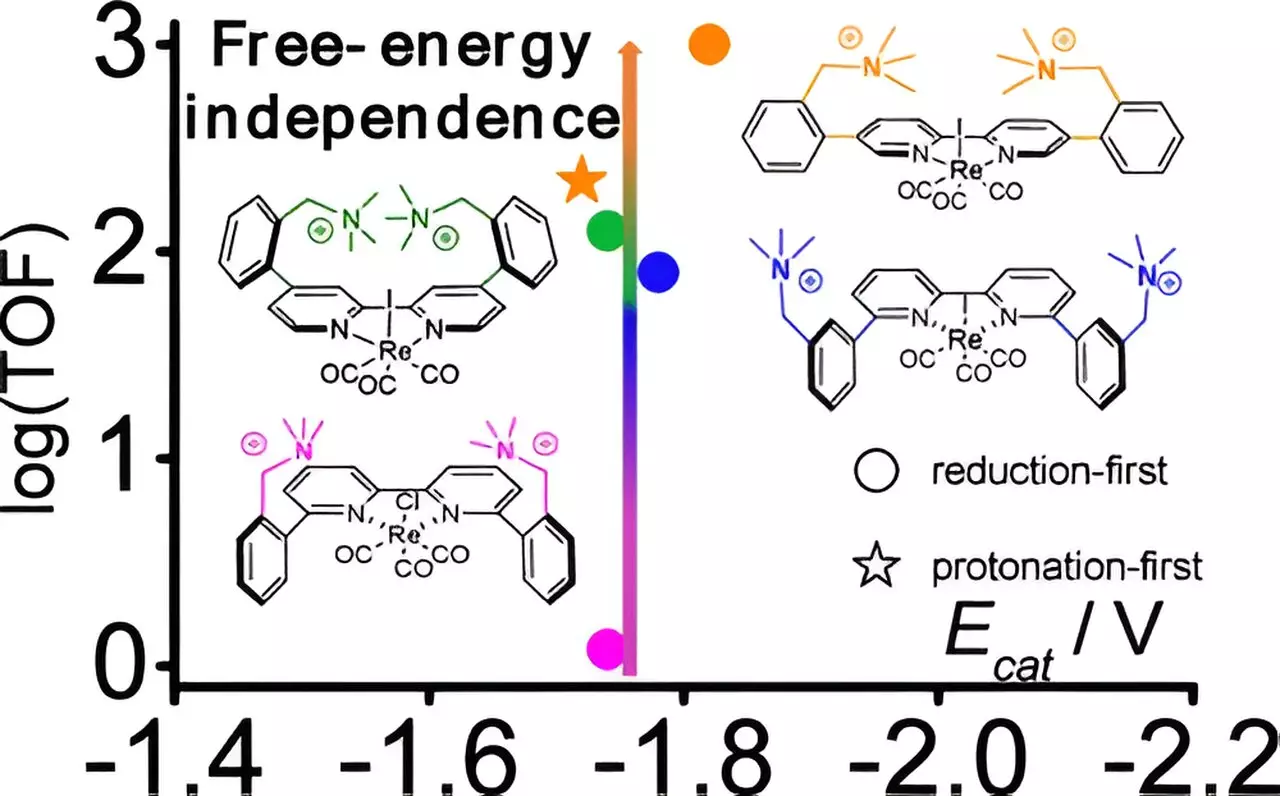
To tackle this challenge, researchers centered their investigation on an existing rhenium-based catalyst. The innovative approach involved strategically modifying this catalyst through the introduction of positively charged molecules, known as cations. By meticulously adjusting the distance between these cations and the core rhenium atom, the research team made a remarkable discovery: at a critical distance, the efficiency of the catalyst surged by an astonishing factor of 800, all achieved without substantially raising the electrical energy input.
This breakthrough underscores the importance of geometric arrangements in fine-tuning catalytic responses. Prior investigations had touched on various methods to improve catalyst properties, but this finding distinctly emphasizes that even minor adjustments in the catalyst’s framework can unlock exponentially higher rates of activity.
The success of this research was significantly bolstered by computational chemistry tools. These technologies not only guided the research team in predicting the effects of their modifications but also elucidated the mechanisms underlying the observed improvements in catalytic performance. By employing resources from the Center for Functional Nanomaterials, the researchers further clarified how the added cations stabilize reactions occurring after the initial catalytic step, leading to the discovery of a low-energy pathway that was previously not recognized in rhenium-based molecular catalysts.
Additionally, various experimental techniques enhanced the research’s credibility. Cyclic voltammetry and infrared spectroelectrochemistry provided vital data, enabling scientists to observe and measure reaction rates and the dynamic structural changes throughout the catalytic process.
A Future Unified with Light-Driven Catalysis
Looking forward, the research team plans to build on their findings by incorporating semiconductor light absorbers—such as silicon—into their catalytic system. Harnessing solar energy through these materials could further diminish the reliance on direct electrical energy for the CO2 conversion process, creating a more sustainable and efficient model. This progression aligns seamlessly with the objectives of the Center for Hybrid Approaches in Sustainable Energy (CHASE), which emphasizes the role of sunlight as a transformative energy source in facilitating liquid fuel generation via CO2 and water transformation.
The exploration of electrochemical reduction methods to mitigate CO2 emissions not only brings attention to pressing environmental challenges but also showcases the immense potential of innovative catalyst design. The collaborative spirit of research, as demonstrated by Brookhaven and its partners, presents a beacon of hope in the quest for sustainable energy practices. As the field of electrochemical catalysis evolves, every advancement—no matter how incremental—holds the promise of moving society toward greener technologies and energy systems, illustrating that even the smallest changes can result in monumental progress. This work reaffirms the potential of novel research methodologies in addressing one of the most significant challenges of our time.

Leave a Reply