Carboxylic acids represent a pivotal class in organic chemistry, integral not only in various natural processes but also fundamental to the pharmaceutical industry. They form the backbone of numerous drugs, such as the widely-used analgesics aspirin and ibuprofen. This versatility stems from their functional groups, which allow them to participate in a variety of chemical reactions. However, as we strive to enhance their efficacy and specificity, introducing elements like fluorine becomes crucial. Fluorinated compounds often exhibit improved biological activity, making them a significant focus in drug design.
The Challenge of Fluorination: A Complex Terrain
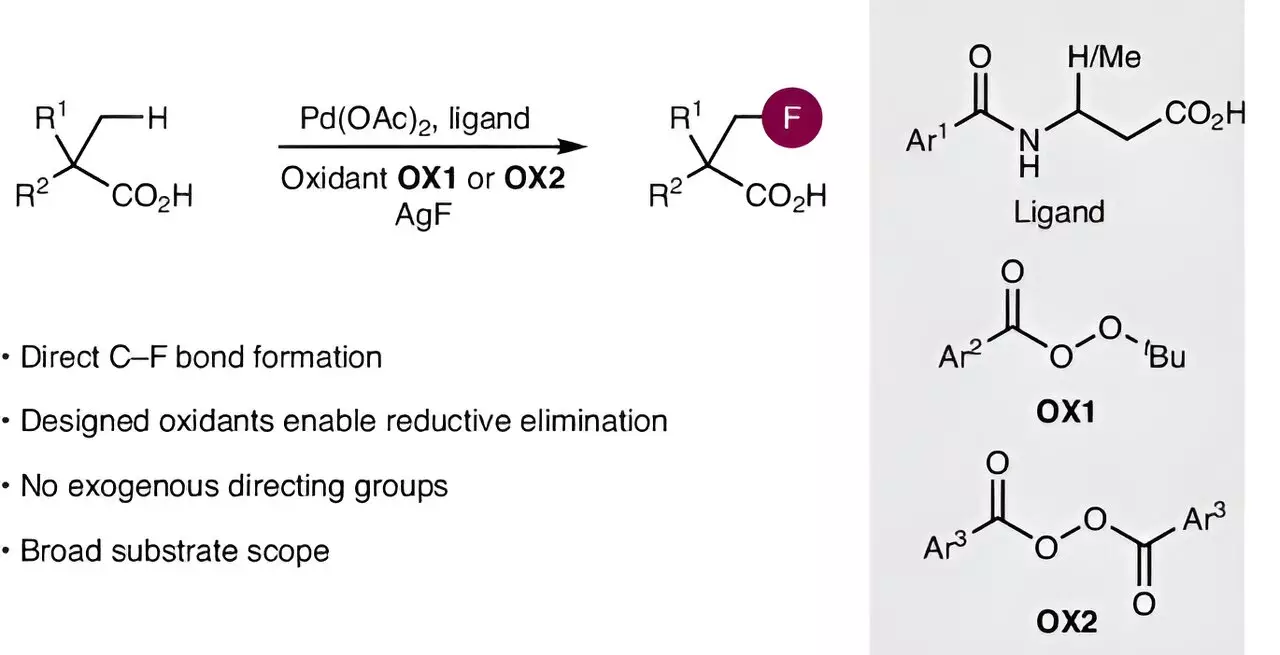
Historically, the introduction of fluorine atoms into carboxylic acids has posed significant challenges. This process usually necessitates complex and multi-step reactions that make it both labor-intensive and time-consuming. The primary difficulty lies in activating the carbon-hydrogen (C-H) bonds within these molecules. Traditional approaches have struggled with these unreactive bonds and the subsequent formation of stable carbon-fluorine (C-F) bonds, which are vital for enhancing the properties of the resulting compounds.
A Game-Changer: Innovative Catalysts
Recent advancements published in Nature Synthesis by an international team of researchers from the Otto Diels Institute of Organic Chemistry at Kiel University, led by Professor Manuel van Gemmeren, mark a pivotal moment in this field. Their research introduces a novel method for installing fluorine atoms directly into aliphatic carboxylic acids, significantly simplifying what was once an arduous process. By utilizing a sophisticated palladium catalyst, the team successfully activated the stubborn C-H bonds in these acids. This activation is a fundamental leap, as effective C-H activation enables the selective incorporation of fluorine, opening up a wealth of possibilities in synthetic chemistry.
Innovative Approaches: Understanding the Reaction Pathway
The researchers didn’t stop at just optimizing catalysts. They meticulously designed a new kind of oxidizing agent aimed at facilitating the critical step of forming the C-F bond. This innovation led to the discovery of an unconventional reaction pathway that further distinguishes their work. The synergy between the new catalyst and the oxidant highlights a sophisticated understanding of reaction dynamics, showcasing how specific design choices can yield more efficient and effective synthetic routes. This approach not only promotes efficiency but also enhances the selectivity of the reactions—an essential factor in pharmaceutical development where precision is paramount.
Implications for the Future of Pharmaceutical Research
The implications of this groundbreaking method extend beyond mere academic interest. The ease and efficiency of directly fluorinating complex carboxylic acids could drive a new wave of drug development and design. Fluorinated compounds have shown great promise in treating various medical conditions, and this novel technique could significantly accelerate the synthesis of new, effective medications. Professor van Gemmeren’s optimism regarding its application potential speaks volumes; as the pharmaceutical sector continually seeks innovative solutions, this method stands poised to unveil new therapies, pushing boundaries in the fight against a myriad of diseases.
By revolutionizing the approach to fluorination, this research could not only enhance the arsenal of chemists and pharmaceutical developers but also contribute to more effective healthcare solutions worldwide.

Leave a Reply