The journey from concept to market for a new drug is labyrinthine and fraught with hurdles. Developing a novel pharmaceutical can stretch across several years, consuming millions of dollars before it even sees a clinical trial. The sobering reality is that over 90% of drug candidates fall short during clinical testing, with a significant number never reaching that crucial phase. While many factors influence a drug’s success, safety remains paramount. This pressing challenge has galvanized researchers to explore new methodologies—enter artificial intelligence (AI)—which holds the promise of transforming early drug discovery and enhancing safety evaluations.
AI Models Paving the Way for Safer Drugs
A significant advance in this domain comes from researchers at the Broad Institute of MIT and Harvard, where AI models are being harnessed to forecast the biological impacts of drugs pre-emptively. Srijit Seal, a prominent figure in the Carpenter-Singh Lab, has developed multiple predictive machine learning tools focused on identifying chemical and structural features of drugs that may pose safety risks to humans. These tools provide critical insights into various biological endpoints relevant to drug developers, including cellular health, pharmacokinetic behavior, and the functionality of vital organs such as the heart and liver.
The innovative AI technologies introduced by Seal aren’t merely about replacing existing laboratory experiments; they act as a guiding compass. By helping to sift through a multitude of potential drug candidates, they allow researchers to concentrate resources on the most viable options, ultimately leading to a more efficient and effective drug development process.
Unearthing Toxicity Through Structure and Data
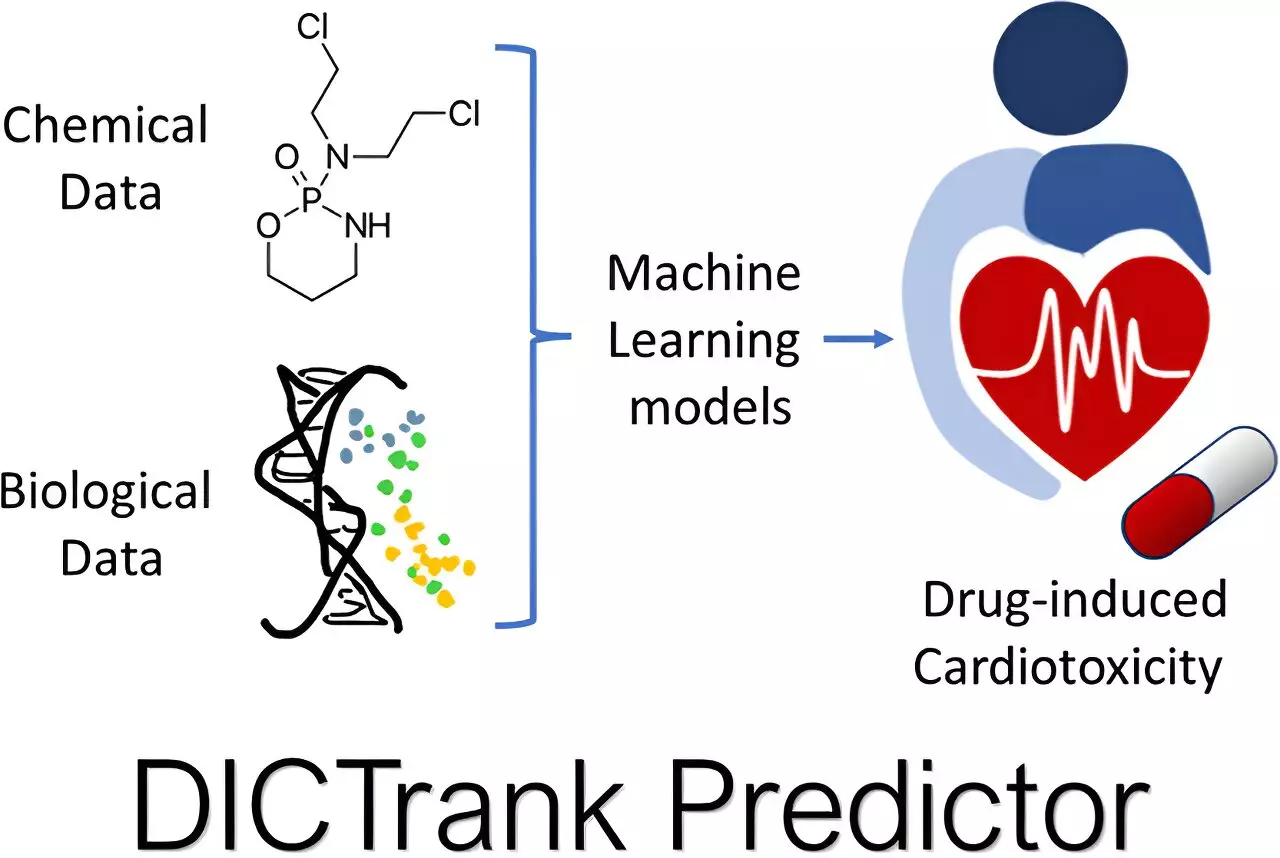
Seal’s foundational work centered on whether more comprehensive toxicology insights could emerge from a drug candidate’s chemical structure. The issue of drug toxicity doesn’t just emerge preclinical; it can manifest even after receiving FDA approval, as evidenced by incidents such as drug-induced cardiotoxicity (DICT) and drug-induced liver injury (DILI), which significantly overlap with post-market withdrawals.
Harnessing the FDA’s carefully curated datasets of toxicological outcomes, Seal identified a pioneering avenue for machine learning applications. He constructed toxicity-predictive models, one focused explicitly on cardiotoxicity and another dedicated to liver injury. Armed with additional data on chemical structure and pharmacokinetic parameters, these models adeptly learned to discern features contributing to drug toxicity.
A noteworthy achievement from this effort is the DICTrank Predictor, a groundbreaking model that correlates with the FDA’s DICT ranking. Furthermore, the DILIPredictor seeks to navigate the intricate challenge of differentiating drug toxicity across species—a vital consideration, given the variances in how similar compounds interact with biological systems.
The Pharmacokinetics Equation
Another cornerstone of Seal’s research endeavors deals with pharmacokinetics or the study of how drugs are absorbed, distributed, metabolized, and excreted within the organism. Understanding these parameters is essential, as drugs that fail to target their intended destination or remain in the body excessively can lead to undesirable outcomes. While traditional modeling in pharmacokinetics is often laden with lengthy processes and expensive instruments, Seal’s predictive machine learning framework may offer a pathway to streamline this assessment.
Through collaborations, he is crafting a predictive model for pharmacokinetics, one that, if successful, will rival existing industry tools. “In pharmacokinetics, integrating machine learning can significantly accelerate the speed at which we move through early-stage assessments, enabling researchers to ‘fail faster’ and concentrate on the most promising pathways,” Seal noted.
Deciphering Cellular Health Through Advanced AI
Beyond toxicity predictions, understanding how drugs affect cellular health remains a pivotal area of concern. When AI models suggest a potential negative impact on cells, biologists often seek additional insights that clarify the underlying mechanisms at play. Seal turned to an open-source imaging software, CellProfiler, which analyzes the visual data of cells, to gain deeper biological interpretations of cellular responses.
However, the challenge emerged when researchers struggled to translate CellProfiler’s image-derived data into applicable biological knowledge. To bridge this gap, Seal developed BioMorph, a deep learning model that synthesizes imaging data from CellProfiler with metrics on cellular health, like growth and replication rates. Through dual training on diverse datasets, BioMorph can elucidate the specific ways in which a drug’s mechanisms may influence cell vitality.
This fusion of AI and biological reasoning stands to empower scientists, offering a robust tool that clearly correlates compounds with their effects on cellular features. BioMorph not only offers predictive capabilities but also fosters a comprehensive understanding that aligns scientific insights with biological realities.

Leave a Reply