Cholesterol is often vilified for its association with heart disease, yet it is an essential molecule that plays a pivotal role in the structural integrity and functionality of cell membranes. Recent research from Rice University, spearheaded by Professor Jason Hafner, sheds light on this complex molecule and its interactions within biomembranes. Published in the Journal of Physical Chemistry, this study challenges existing paradigms and opens new avenues for understanding diseases where membrane organization is crucial, such as cancer. By deciphering cholesterol’s role, researchers aim to illuminate potential therapeutic targets that could mitigate membrane-related diseases.
Understanding how cholesterol operates within cellular membranes has always posed a significant challenge for researchers. Traditional methods often fell short of providing a comprehensive picture, mainly due to the intricate arrangements of lipids and proteins within these membranes. Hafner’s team turned to a cutting-edge technique called Raman spectroscopy, which utilizes laser light to provide molecular insights. This approach enabled the researchers to examine cholesterol molecules in their natural habitat — the membranes themselves.
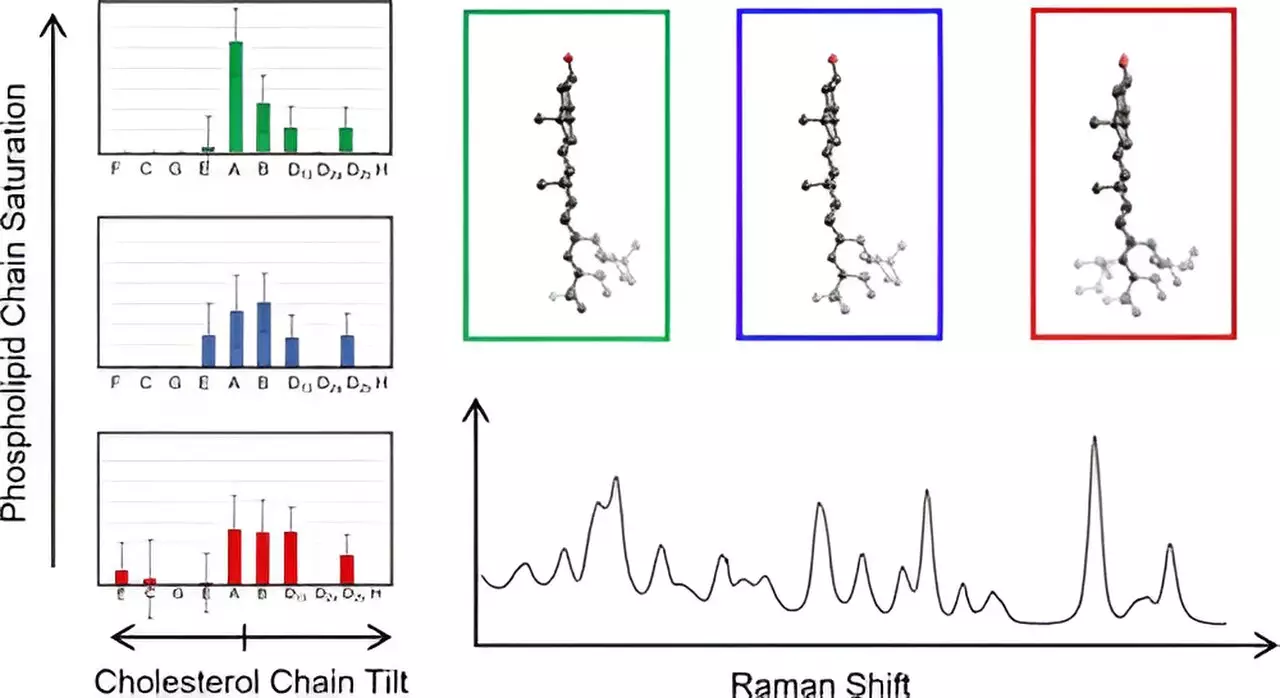
By leveraging Raman spectroscopy, the team was able to generate vibrational spectra, which reveal detailed information about the molecular structures at play. This was further enhanced by employing density functional theory (DFT), a quantum mechanical modeling method that allowed them to correlate experimental findings with theoretical predictions. Such a multi-faceted approach not only validated their measurements but also refined their understanding of cholesterol’s architecture within biomembranes.
One of the study’s groundbreaking findings was the identification of previously unknown structural variations among cholesterol molecules. The researchers cataloged 60 distinct cholesterol structures, focusing on the unique fused ring and carbon chain configurations. They discovered that these structures could be classified based on how the carbon chain deviated from the ring plane, heralding a new understanding of cholesterol’s diverse roles in cell membranes.
Perhaps most remarkably, they found that cholesterol molecules grouped together exhibited identical low-frequency spectra, simplifying the analysis considerably. “This allowed us to simplify the analysis and fit our experimental data to map out membrane cholesterol chain structures,” noted Hafner. Their observations mark a significant advancement in biophysical chemistry, as this is the first instance of directly measuring cholesterol chain structures in their actual membrane environment.
The implications of this study extend beyond a mere academic exercise; they could fundamentally alter how scientists approach diseases linked to membrane dysfunction. Understanding cholesterol’s intricate role may lead to novel treatments for conditions such as cancer, where membrane integrity is vital.
With contributions from various researchers, including physics graduate student Kyra Birkenfeld and bioengineering undergraduate student Tia Gandhi, this multi-disciplinary effort illustrates the importance of collaborative research in tackling complex biomedical questions. As future studies build upon Hafner’s findings, the hope is that we can unlock the mysteries of membrane organization and its relation to health and disease, ultimately leading to innovative therapeutic strategies.

Leave a Reply