As the world grapples with sustainable solutions to meet its growing consumption demands, propylene has emerged as an essential player in the arena of synthetic materials. This versatile petrochemical is not just a component of various plastics, including polypropylene food containers and medical devices, but also a vital ingredient in many industrial processes. With escalating global demands, the race to find an efficient and eco-friendly method for propylene production is more pressing than ever. The catalyst for this change may lie in a groundbreaking study from researchers at the U.S. Department of Energy’s Argonne and Ames National Laboratories, which promises to shift traditional production paradigms significantly.
The Conventional Production Bottleneck
Traditionally, the conversion of propane to propylene involves utilizing catalysts such as chromium or platinum, which are either toxic or prohibitively expensive. The existing methodologies also require high operational temperatures, leading to a spike in energy use and an increase in carbon emissions. Given that these emissions account for a staggering 80% of the greenhouse gases released in the United States, the need for alternative techniques is imperative. The implications of continuing along this path are alarming; as demand rises, so does the environmental burden of traditional propylene production methods.
A Fresh Perspective: Zirconium and Silicon Nitride
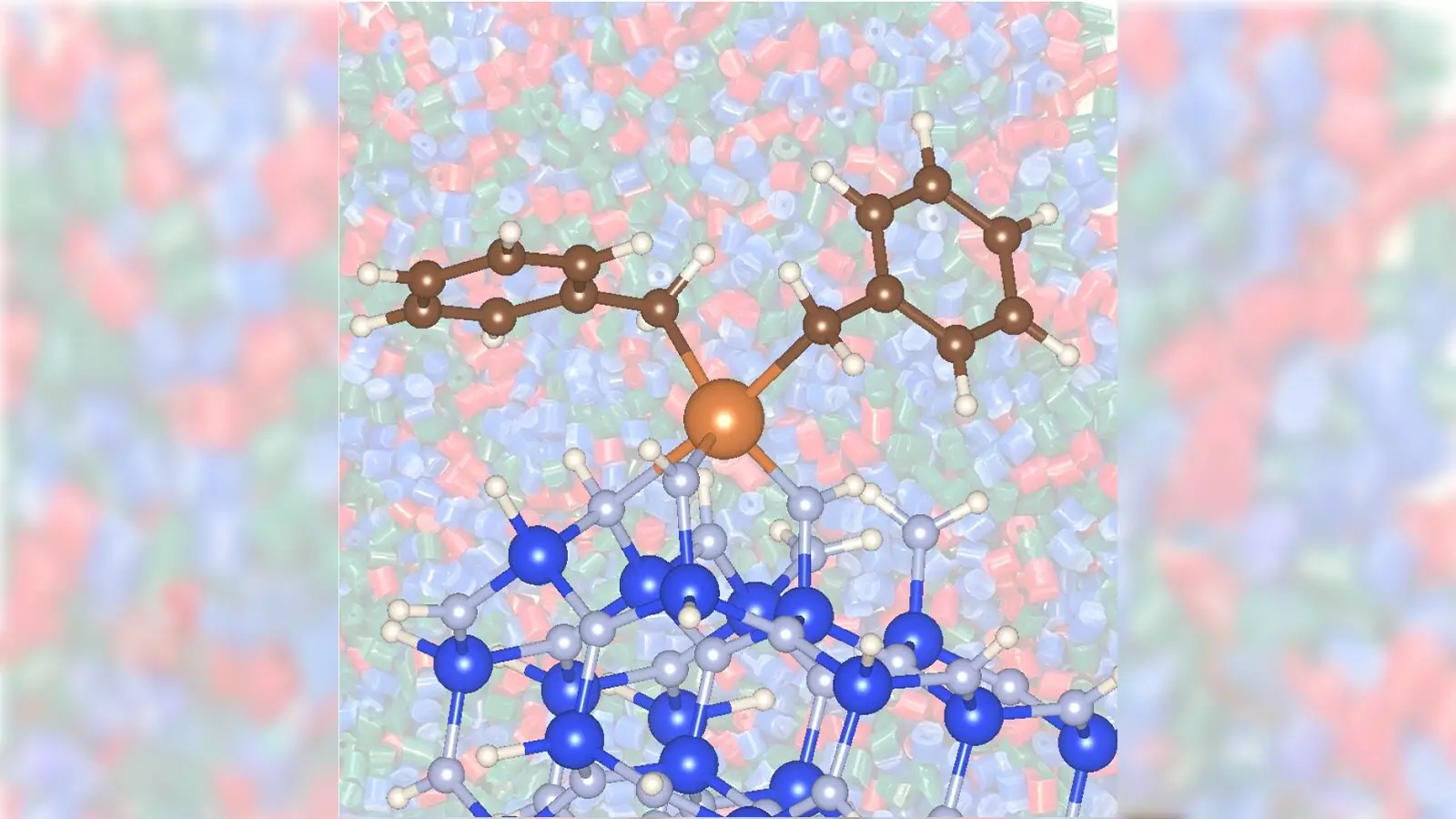
What the researchers at Argonne and Ames have introduced is nothing short of a revolution. By combining zirconium with silicon nitride, they created a new catalyst that is not only more efficient but also less toxic. This innovative combination allows for the conversion of propane to propylene at significantly lower temperatures—842 degrees F compared to the standard 1,022 degrees F employed with traditional methods. This remarkable reduction in temperature translates directly into lower energy costs and fewer greenhouse gas emissions.
Furthermore, the silicon nitride support material enhances the activity of zirconium in ways that conventional oxide materials simply cannot. The research team discovered that the zirconium/silicon nitride catalyst increased reaction speeds, presenting a faster route to produce propylene and leaving traditional approaches in the dust. The study underscores the importance of looking beyond traditional materials to explore how different combinations can lead to enhanced catalytic processes.
Significance of the Research Findings
This study sheds light on the potential for other low-cost metals to be used in similar catalytic processes, opening doors to sustainable solutions across various chemical transformations. As principal researcher David Kaphan highlighted, this research reveals a “window into nitride-supported metal reactivity.” The insights gained suggest that expanding the material palette for catalytic agents can significantly reduce environmental footprints while meeting industrial demands. It’s a promising revelation that could change the landscape of chemical manufacturing as we know it.
Moreover, the collaborative nature of this research emphasizes a pressing point: multidisciplinary approaches are essential for scientific breakthroughs. Each researcher brought distinct expertise, contributing to a comprehensive understanding of the chemical processes at play. As Fredéric Perras from Ames National Laboratory acknowledged, the knowledge of the silicon nitride surface’s composition remains largely unexplored—a realm filled with potential.
The Future of Catalyst Research
The implications of this research extend beyond just propylene production. The techniques and understandings developed here could be applied to various other catalytic reactions, making it a blueprint for future environmentally-conscious innovations in chemistry. The success of this study drives home the importance of continuing to explore nontraditional materials in a quest for more efficient chemical reactions.
Moreover, in an age where sustainable practices are vital for the health of our planet, such findings create a ripple effect—encouraging industries to rethink material choices and production methods. Propylene production may be just the tip of the iceberg; this ingenuity could spark a broader movement towards sustainable practices in entire sectors.
The research published in the Journal of the American Chemical Society serves as a landmark achievement, fortifying our understanding of catalysis while aligning with global energy and environmental aspirations. As the world begins to embrace greener technologies, the innovations uncovered by these passionate scientists at Argonne and Ames offer an exhilarating glimpse into a more sustainable and economically viable chemical future.

Leave a Reply