With the increasing industrialization and urbanization around the globe, water sources become vulnerable to pollution from heavy metals such as cadmium and lead. The ingestion of water infused with these toxic metals can have profound health implications for both humans and aquatic ecosystems. Heavy metals can accumulate in living organisms and create various long-term health problems, ranging from neurological damage to cancer. Traditional methods of water purification, such as filtration techniques and activated carbon, often fail to provide an effective solution and can be resource-intensive. Recent research, however, highlights an innovative approach to tackling this pressing issue using sugar-derived polymers.
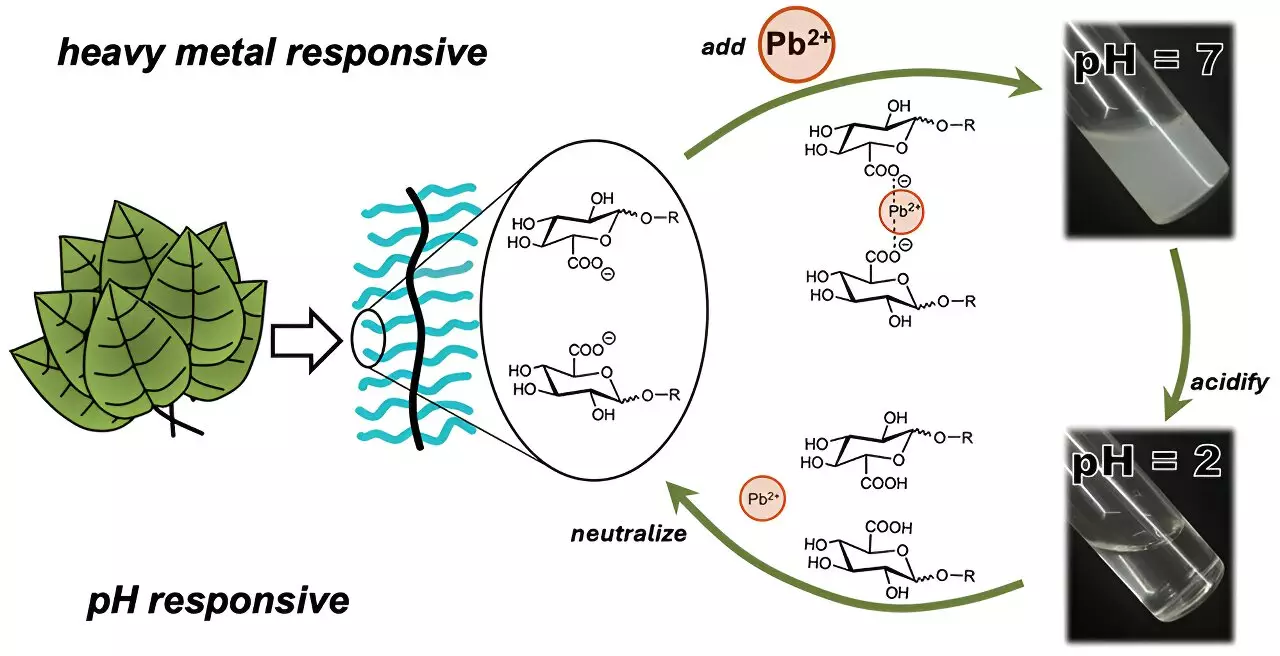
Polymers derived from natural sources, especially plants, have garnered attention due to their inherent ability to interact with heavy metal ions. These polysaccharides, primarily composed of sugar molecules structured into macromolecular chains, form a protective barrier for plant cells and can bond with pollutants, making them invaluable in environmental remediation. However, a significant hurdle remains—many polysaccharides are soluble in water, necessitating the use of additional agents to create insoluble structures capable of metal ion capture. This often complicates the purification process and can introduce further pollutants into the water.
Enter the groundbreaking research led by Cassandra Callmann and her team at the University of Texas at Austin. They have successfully synthesized a new type of polymer, which not only possesses sugar-like characteristics but also has adjustable water solubility, allowing for efficient metal ion trapping while minimizing environmental footprint.
The researchers crafted a series of polymers featuring a sturdy, water-insoluble backbone to withstand various water conditions. Attached to this backbone were strategically engineered water-soluble carbohydrate “charms.” These charms were designed to bind effectively with heavy metal ions, particularly cadmium and lead. During preliminary tests, a specific carbohydrate charm containing a carboxylic acid functional group was found to exhibit superior binding capabilities with cadmium ions.
Remarkably, after a mere three minutes of exposure to water spiked with cadmium, the polymer began forming visible clumps, facilitating straightforward removal through filtration. Additionally, the clumping mechanism is reversible; by altering the water’s acidity, the polymer can release the bound cadmium for potential reuse. This feature underscores the material’s promise not only as an effective metal trap but also as a recyclable solution for water treatment.
To further validate their innovations, the research team conducted tests using water samples from the Colorado River, compellingly demonstrating the effectiveness of their polymer in a real-world scenario. Despite the presence of competing metal ions like calcium, sodium, and magnesium, the polymer successfully captured 20% of cadmium and a staggering 45% of lead over a 24-hour period. These findings are significant given the complex nature of natural water sources, where multiple contaminants typically co-occur.
The capability to selectively capture heavy metals while ignoring other harmless ions is particularly noteworthy. It opens doors to the development of specialized water treatment facilities and modular systems that can adapt to varying pollutant loads in different water bodies.
The advent of this new sugar-derived polymer signals a promising shift toward more efficient, sustainable, and environmentally friendly water purification technologies. As water scarcity and contamination continue to pose global challenges, finding viable and innovative methods for heavy metal removal is crucial. By utilizing natural polymers with built-in tunability, researchers and industries alike could minimize energy expenditure, reduce reliance on toxic chemicals, and enhance the overall sustainability of water treatment processes.
The research spearheaded by Callmann and her team represents a crucial step towards solving heavy metal contamination in our water systems. Through further investigation and scaling of this technology, there is potential for transformative changes in how we safeguard important freshwater resources.

Leave a Reply