Bacteria exhibit a fascinating array of strategies to ensure their survival in hostile environments, and one of the most effective is the formation of a protective capsule. This article delves into the intricate mechanisms behind bacterial capsules, their structure, and their potential as targets for therapeutic interventions and biotechnological advancements.
Bacterial capsules are intricate structures composed of various polysaccharide chains that surround the bacterial cell wall. These capsules serve multiple vital functions; chief among them is their role as a protective barrier. By encasing themselves in this sugary sheath, bacteria can shield themselves from physical stressors and dehydration, which are commonplace in their habitats. Additionally, the capsule acts as a cloaking device, rendering the bacteria invisible to the immune system. This stealth capability significantly enhances the pathogenicity of bacteria, allowing them to thrive and proliferate within the human body.
Current research indicates that disrupting the formation of capsular polymers would notably diminish the virulence of bacterial pathogens. As a result, the enzymes responsible for synthesizing these capsules are emerging as promising targets for new pharmacological strategies. This paves the way for innovative treatments that could potentially outmaneuver antibiotic resistance—an escalating global health crisis.
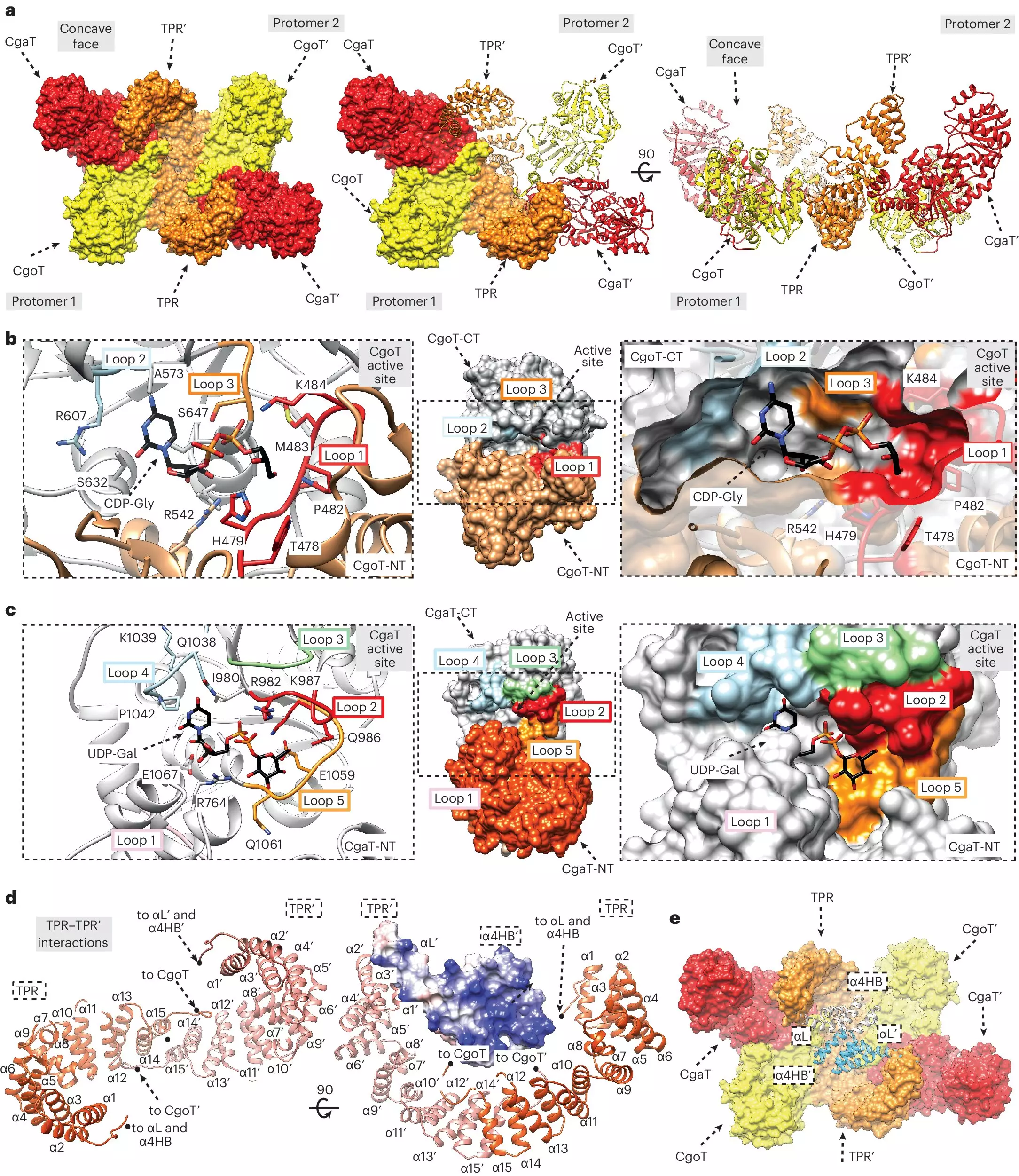
Despite their crucial role in bacterial virulence, the specific mechanisms by which capsular polymers are anchored to the bacterial membrane remain poorly understood. Researchers from the Hannover Medical School, led by Dr. Timm Fiebig, have made significant strides in elucidating this enigma. By identifying the ‘linker’ that connects the capsule to the membrane, they have illuminated a key piece of the biosynthetic puzzle.
The membrane itself contains fatty acid molecules serving as anchors, but the research team has isolated the intermediate linker that connects these anchors to the capsular polymers. This discovery is invaluable, as it opens up avenues for developing new antibacterial agents that could disrupt this critical link, rendering the bacteria susceptible to immune attacks. Moreover, characterizing the enzymes known as transition transferases, which produce this linker, could revolutionize the way we approach vaccine development.
Central to the formation of bacterial capsules are the capsular polymerases responsible for synthesizing polysaccharide chains. Dr. Fiebig’s team has not only characterized the linker but also clarified how the capsular polymerases interact with it. Their findings reveal that these polymerases can extend the linker, facilitating the construction of longer sugar chains. Such configurations likely enhance the bacteria’s ability to evade immune detection.
Utilizing specialized chromatography techniques, the researchers were able to purify the enzymes and their corresponding linkers for further structural analysis. Their experiments confirmed that transition transferases, in conjunction with capsular polymerases, contribute to the production of longer sugar chains, ultimately leading to a more robust protective capsule. This insight builds upon previous work that demonstrated similar mechanisms in bacteria like Haemophilus influenzae type b (Hib), which is implicated in serious respiratory infections.
The implications of these findings extend beyond basic scientific inquiry; they hold the potential for significant biotechnological applications. By manipulating the enzymes involved in capsular synthesis, researchers can engineer vaccines and therapeutics that could combat a broad spectrum of bacterial infections.
The recent study has also highlighted the conservation of transition transferases across different bacterial species, suggesting that targeting these enzymes could yield broad-spectrum antibacterial drugs. This approach could offer a promising alternative to traditional antibiotics, particularly in an era where antibiotic resistance is becoming a major public health threat.
While the current findings shed light on the mechanisms of capsular formation, they also open the floor for further research. For instance, understanding the structural differences between different types of linkers and capsules could lead to targeted strategies against various bacterial strains. With researchers observing similarities in linker structures among pathogens that cause serious conditions such as meningitis and urinary tract infections, the future of bacterial research may hold the key to effectively disabling these harmful microorganisms.
The ongoing investigation into bacterial capsules and their synthesis is of paramount importance for both fundamental research and therapeutic innovation. By disabling the protective mechanisms that aid bacterial survival, we may be on the cusp of a new generation of treatments that could significantly improve patient outcomes in infectious diseases. The work of Dr. Fiebig and his team not only provides a deeper understanding of bacterial adhesion and virulence but also lays the groundwork for next-generation vaccines and antibacterial agents that could combat some of the world’s most resilient pathogens.

Leave a Reply