When we think about ice, it is often viewed as a solid, nearly unchangeable entity, encasing water in a frozen state. However, this perception neglects a crucial aspect: the dynamic interaction that occurs at the interface between ice and liquid water. This fluid relationship is vital for a variety of phenomena we experience in everyday life, from the tricky nature of ice skating to the enticing melting of ice cream on a warm day. Recognizing how ice behaves in the presence of liquid water opens up discussions about not just physical interactions but also potential applications in science and technology.
Recent breakthroughs by researchers at Kobe University and the Institute for Molecular Science have provided us with a transformative perspective on these interactions. Armed with advanced technology, including a refrigerated microscope and antifreeze, they have shed light on the elusive layer that exists between solid and liquid states. Understanding this interface involves intricate details that science has struggled to capture, but new methodologies reveal the surface dynamics of ice with unprecedented clarity.
The Challenges of Direct Observation
Previous studies have attempted to comprehend the properties of ice in relation to water, but inherent challenges hindered progress. Ice and water exist in a rapid state of flux; one can easily transform into the other, making direct observation a daunting challenge. The team, led by Onishi Hiroshi, devised an innovative approach to tackle this issue. By using antifreeze to maintain temperatures below the freezing point, they were able to observe the ice without the interference of melting. This creative ingenuity is a testament to human curiosity and determination in the face of nature’s complexities.
However, the journey was not without its hurdles. The scientists faced numerous technical obstacles, particularly in stabilizing their measurement instruments at sub-zero temperatures. The innovation of cooling the entire microscope system was critical in successfully achieving stable readings. This endeavor raises interesting questions about the lengths to which scientists must go to unveil nature’s secrets. Such persistence is not merely a footnote in scientific literature; it reflects the ever-evolving methods driven by human curiosity.
The Surprising Findings
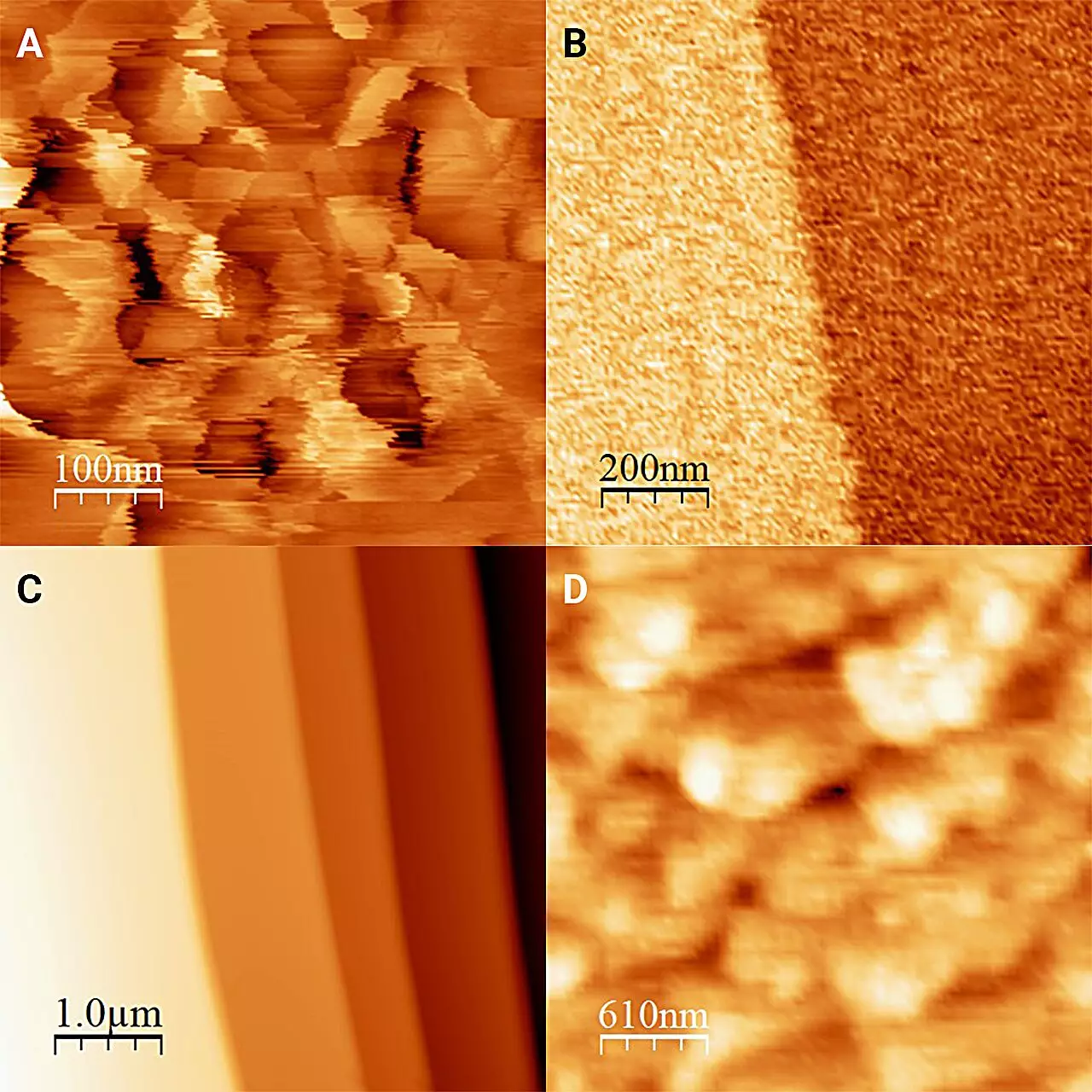
What the researchers ultimately uncovered was revolutionary. Opposed to traditional understanding, they observed that ice in contact with 1-octanol— a type of antifreeze—appeared remarkably flat, with minute raised features only a single molecular layer high. Previously, ice was thought to manifest a more textured surface characterized by frost structures. This revelation provides substantial insights into how various liquids can significantly influence the characteristics of the adjacent ice.
By experimenting with different alcohols, they demonstrated that slight variances in molecular structure and properties could lead to vastly different ice surfaces, underpinning the importance of specific conditions in research. Each liquid not only behaves differently but may engage with ice in a myriad of unforeseen ways. As such, this research is a reminder that the quest for knowledge is often paved with unexpected twists, continually reaffirming that nature often holds surprises even when we think we’ve understood it.
Rethinking Ice’s Properties
In addition to the disparity in surface structure, another fascinating result emerged: the peculiar “hardness” of ice. The team’s investigations showed that under the presence of specific liquids, ice is much harder than what prior research had indicated. This new understanding has implications that may stretch far beyond theoretical realms; insights into the hardness of ice can affect various fields, from engineering to environmental science.
One can only imagine the breadth of applications that could arise from a deeper understanding of ice’s properties, especially in a world increasingly challenged by climate change. The potential for new materials or techniques to adapt to slowly shifting environments seems vast.
The Path Forward
The findings from this study aren’t merely an academic achievement; they pave a pathway for future exploration. The team’s aspiration to enhance the resolution of their measurements to that of single water molecules signals an exciting frontier in molecular science. As researchers refine their methods, we may witness developments that can tangibly impact our understanding of ice and liquid interactions. The methodologies employed here exemplify a broader lesson in the scientific process—a reminder that success often hinges on perseverance, creativity, and unyielding curiosity in uncovering nature’s intricate layers.

Leave a Reply