The ongoing discussion surrounding climate change has catalyzed a significant interest in sustainable technologies, particularly in the conversion of carbon dioxide (CO2) into valuable chemicals. For years, researchers have pursued methods to transform CO2 emissions into useful resources, paving the way toward a more sustainable and circular economy. However, one of the persistent challenges in carbon dioxide reduction processes lies in optimizing selectivity—ensuring that the reactions yield desired products efficiently.
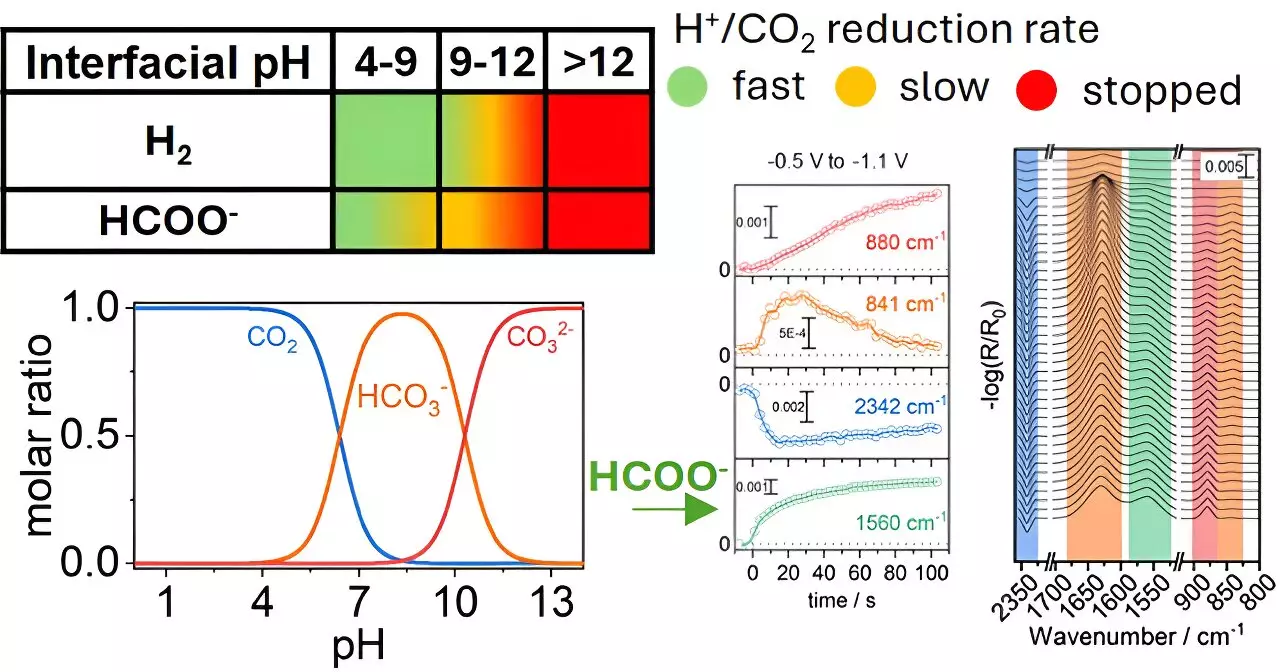
A recent study conducted by researchers at the University of Twente, under the leadership of Georgios Katsoukis, has shed light on a critical aspect of this process: the chemical environment surrounding copper electrodes. The findings, published in the journal *ACS Catalysis*, reveal that manipulating local conditions, such as pH levels, can significantly impact the efficacy of converting CO2 into formate—a chemical with widespread industrial utility.
Traditionally, most research efforts have fixated on the catalytic material itself when exploring ways to enhance CO2 reduction. However, this study challenges that notion by showcasing the underappreciated role of the aqueous environment that envelops these catalysts. By examining how variations in pH affect the reaction dynamics at copper electrodes, the research team established that these local chemical conditions dictate not only the speed but also the efficiency of the conversion process.
Selectivity remains a formidable obstacle in CO2 reduction chemistry. Various products can emerge from the same reaction pathway, dependent on environmental parameters and reaction conditions. This research highlights a paradigm shift in focusing not solely on the catalyst but also on precisely tuning the chemical milieu surrounding it. Such insight could lead to enhanced selectivity towards formate and potentially extend the operational lifespan of the electrodes involved, presenting an innovative approach to catalytic design.
The implications of Katsoukis and his team’s work extend beyond a singular study. They propose a roadmap for future endeavors in CO2 reduction technologies. By integrating strategies that optimize both catalyst material and the adjacent chemical environment, scientists may make substantial strides toward crafting more efficient systems for converting CO2 emissions into commercially valuable products.
As the pressure to address climate change mounts, understanding the nuanced interaction between chemical environments and catalytic processes may serve as a key that unlocks the full potential of CO2 utilization. This could herald an era where we not only capture greenhouse gases but also transform them into resources that support a sustainable future.
Advancing CO2 reduction technologies through environmental engineering presents a compelling opportunity, pushing the boundaries of what is possible in sustainable chemistry. With researchers keenly exploring these novel avenues, society may soon reap the benefits of innovative approaches to combat climate change and foster a circular economy.

Leave a Reply