The potential of utilizing carbon dioxide (CO2) as a raw material for the synthesis of valuable chemicals has garnered significant attention in recent years. This is particularly true in light of the ongoing climate crisis, as harnessing CO2 offers a dual benefit: reducing greenhouse gas emissions and providing essential building blocks for a range of products, including fuels and plastics. Recent advances in spectroscopic techniques and theoretical modeling have propelled the understanding of the electrochemical reduction of CO2 (CO2RR), particularly in the production of high-demand chemicals like ethylene and ethanol. Studies such as the one published in Nature Energy underscore the remarkable progress made in this field.
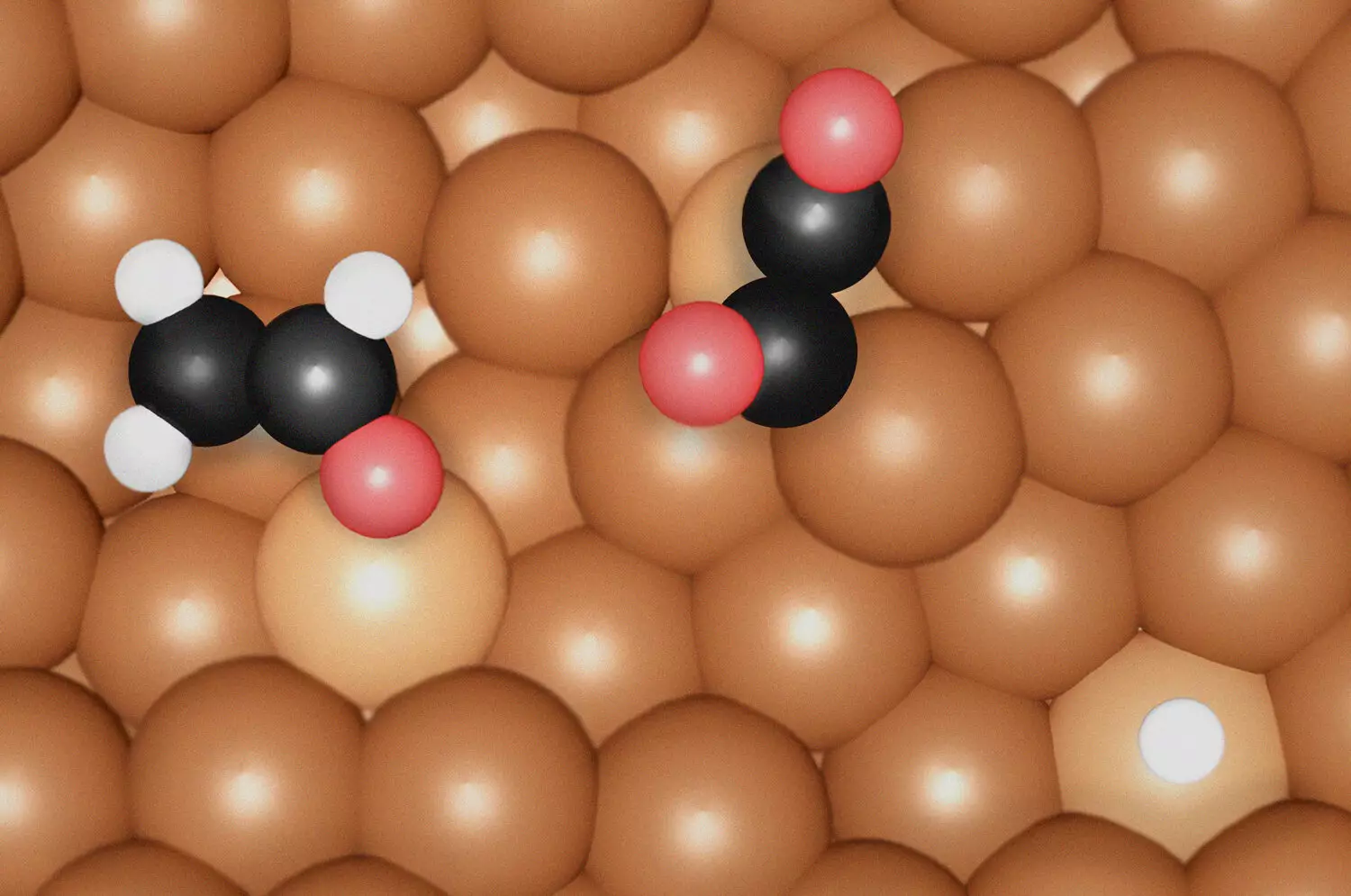
The study titled “Key Intermediates and Cu Active Sites for CO2 Electroreduction to Ethylene and Ethanol” offers groundbreaking insights into the mechanisms behind CO2RR. While CO2 can theoretically be transformed into various chemicals, the intricacies of these pathways have baffled researchers for years. Understanding these mechanisms is critical for the rational design of catalysts that can efficiently drive these reactions. In this research, the authors explore the specific roles of copper (Cu) active sites during the reduction of CO2, whereby the formation of ethylene is tied to particular intermediates that occur at undercoordinated Cu sites.
Contrarily, the synthesis of ethanol necessitates a vastly different environment and a distinct key intermediate known as *OCHCH2. This discovery highlights the nuanced nature of the CO2RR pathways and emphasizes that the catalytic environment, specifically how the Cu sites are coordinated, plays a vital role in determining which chemical products are favored during the reduction process.
Employing sophisticated tools like in-situ surface-enhanced Raman spectroscopy (SERS) and density functional theory (DFT), the researchers gained unprecedented insights into the molecular interactions occurring on Cu electrocatalysts. The integration of experimental data with theoretical modeling facilitated a deeper understanding of how reaction intermediates evolve during CO2RR. This methodological approach provided a robust framework for evaluating the effectiveness of different catalytic sites, marking a significant advancement in the field.
The team’s findings shed light on the correlations between specific surface morphologies and catalytic performance. Notably, they discovered that the irregularities presented at the atomic level on Cu surfaces enhance the binding capability of CO, a crucial step in the electrochemical reduction process. These undercoordinated Cu sites, which may be formed dynamically under reaction conditions, emerged as pivotal contributors to generating ethylene and ethanol efficiently.
The insights derived from this research have far-reaching implications for the chemical industry. By elucidating the intricate conditions conducive to producing ethylene and ethanol selectively, researchers can introduce new strategies for catalyst design tailored to mitigate CO2 emissions. This advancement represents a potential paradigm shift in the sustainability of chemical manufacturing processes, particularly in industries dependent on petrochemical sources for plastics and fuels.
Furthermore, the collaboration between research institutions, combining expertise across theoretical and experimental domains, showcases the importance of multidisciplinary approaches in tackling complex environmental challenges. Such partnerships can spur innovation and accelerate the development of scalable solutions.
As the threat of climate change looms larger, the need for sustainable practices in the chemical industry has never been more critical. Research like that conducted by the Interface Science Department at the Fritz Haber Institute and the Institute of Chemical Research of Catalonia represents crucial advancements in understanding the electrochemical reduction of CO2. By unveiling the key intermediates and active sites essential for producing valuable chemicals, this work lays the groundwork for more efficient catalytic processes that could dramatically reduce the carbon footprint of chemical production.
The evolving understanding of CO2RR signifies not only a scientific breakthrough but also hope for a more sustainable future where environmental concerns and industrial needs can be harmonized. The meticulous research, analysis, and collaborative efforts encapsulate the pioneering spirit needed to combat the pressing challenges of our time, ultimately leading us toward a greener and more sustainable planet.

Leave a Reply