As the world pivots towards renewable energy sources, the reliability of these systems becomes paramount. While solar, wind, hydro, and geothermal energy have the potential to revolutionize our power grids, their inherent variability presents significant challenges. Energy storage solutions that can consistently harness this intermittent supply are crucial for sustainable development. Hydrogen, with its versatility and potential as a clean fuel, emerges as a key player in this energy landscape, particularly through water electrolysis.
Understanding Alkaline Water Electrolysis (AWE)
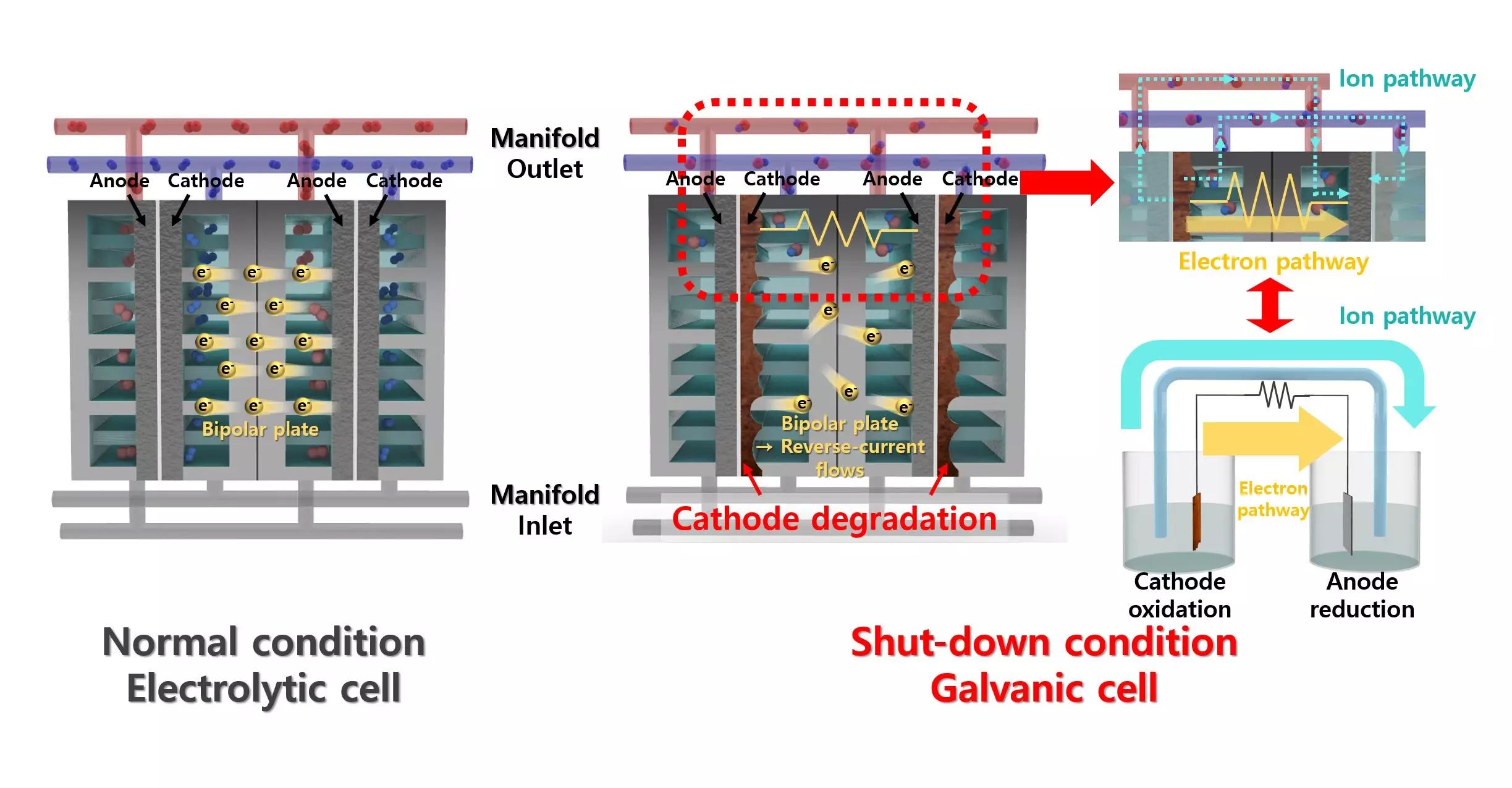
Alkaline water electrolysis (AWE) represents a viable method for hydrogen production. By electrolyzing water with an alkaline solution, AWE systems promise relatively low operational costs and high durability compared to other methods. Nonetheless, these systems are not without their own issues. The production process can suffer from degradation due to reverse currents when power supply is interrupted. This degradation not only affects the lifespan of the electrolyzers but also the overall efficiency of hydrogen production.
Revolutionary Research at the Forefront of Catalyst Development
Recently, a collaborative research effort led by Professor Jeong Woo Han from Seoul National University and involving leading scientists from Pohang University of Science and Technology (POSTECH) has made significant strides in this area. This team, including Professor Yong-Tae Kim, Dr. Sang-Mun Jung, and MSc student Yoona Kim, has developed a catalyst designed to mitigate the adverse effects of reverse current in AWE systems. This innovative catalyst utilizes a simple yet effective coating of lead (Pb) on nickel (Ni), transforming conventional assumptions about catalyst performance in hydrogen evolution reactions.
Lead’s Role in Enhancing Catalyst Performance
While lead is typically eschewed in favor of more active materials due to its historically low performance in hydrogen production, the research showcased its unexpected benefits. By utilizing lead as a co-catalyst, the efficiency of nickel—a well-known hydrogen evolution reaction catalyst—was significantly enhanced. The lead coating promotes critical chemical reactions such as proton desorption and water dissociation, pushing the system’s performance to new heights. This breakthrough reveals a paradigm shift in thinking about catalyst material choices and their potential in sustainable energy applications.
A Game-Changer for Electrolysis Systems
The implications of this work are profound. By demonstrating strong resistance to reverse current through repetitive oxidation reactions, this new catalyst presents a solution to a problem that has long plagued AWE systems. Unlike previous solutions, which often required cumbersome additional equipment to combat reverse current effects, this innovative lead-coated nickel catalyst offers a streamlined, cost-effective approach to enhancing hydrogen evolution efficiency.
Furthermore, Professor Yong-Tae Kim’s declaration of their findings—stating that this is the first study to explore degradation via material solutions in AWE—is not merely academic; it signifies a turning point in hydrogen production technology. The ability to produce hydrogen more efficiently and with greater longevity means that the transition to a hydrogen economy is one step closer to becoming a reality, setting the stage for a more sustainable future.
In the quest for clean energy solutions, this research stands as a beacon of innovation, fostering optimism about the future of hydrogen as a pivotal component in global energy systems.

Leave a Reply