Gallium, an intriguing metal that was first discovered in 1875 by French chemist Paul-Émile Lecoq de Boisbaudran, has always captivated the scientific community with its unusual properties. Known primarily for its remarkably low melting point that allows a gallium spoon to dissolve in a cup of tea, its applications stretch far beyond this remarkable quirk. Gallium plays a crucial role in modern technology, particularly in the realm of semiconductors, which are foundational to the electric devices we rely on today. Its distinctive characteristics—such as existing as ‘dimers’ and its behavior at various temperatures—place gallium firmly in the spotlight of contemporary research.
The Recent Breakthrough: Understanding Gallium’s Atomic Behavior
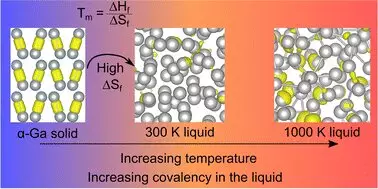
Recent research conducted by scientists at the University of Auckland has unveiled new dimensions of gallium’s atomic structure and behavior, challenging established scientific doctrines. Historically, it was long believed that gallium’s behavior could be explained within the frameworks of conventional metallic bonding. However, findings from a study led by Dr. Steph Lambie and her colleagues reveal a revolutionary truth: gallium’s covalent bonds—typically associated with non-metals—disappear upon melting but reappear at higher temperatures, calling into question decades of accepted scientific understanding.
This research, aptly named “Resolving Decades of Debate: The Surprising Role of High-Temperature Covalency in the Structure of Liquid Gallium,” highlights the necessity of revisiting and reinterpreting the scientific literature surrounding gallium. Professor Nicola Gaston aptly summarizes the astonishment that accompanied these revelations, noting how “thirty years of literature on the structure of liquid gallium has had a fundamental assumption that is evidently not true.” This not only advances our knowledge of gallium itself but also demands a reassessment of our basic concepts in metallurgical science.
The Role of Entropy: A Key to Gallium’s Low Melting Point
Integral to understanding gallium’s peculiar melting behavior is the concept of entropy, signifying disorder within a system. The recent study posits a fascinating correlation between the disappearance of covalent bonds at melting point and a significant rise in entropy. By desiring to decode gallium’s processes, researchers have implicitly spotlighted a key factor influencing not only gallium but possibly other metallic behaviors as well. The re-emergence of these covalent bonds at elevated temperatures suggests a complex layer of atomic interaction that defies traditional assumptions about metallic structures.
This newfound understanding could have wide-reaching implications in advancements in nanotechnology, an area where molecular manipulation is paramount in creating innovative materials. For instance, gallium is instrumental in developing liquid metal catalysts and ‘self-assembling structures’—a cutting-edge technology aiding in the spontaneous organization of disordered materials into functional forms.
Gallium’s Practical Applications: From Technology to Space Exploration
The ramifications of this discovery reach well beyond theoretical science. Gallium’s application in semiconductors is a cornerstone of modern electronics, found in everything from solar panels to high-performance computing devices. Furthermore, its role as an alternative to hazardous materials like mercury in thermometers strengthens its position as a safer choice for various applications.
Interestingly, the potential of gallium extends into the realm of astrobiology. Scientists seeking signs of ancient life on Mars have identified gallium as a possible chemical ‘fingerprint’ that could indicate the historical presence of microbial life. The intersections between gallium’s chemical behavior, its industrial significance, and its potential role in extraterrestrial research reveal an intricate tapestry of possibilities that continue to unfold.
The Enduring Fascination with Gallium
The name gallium itself pays homage to its French roots, deriving from ‘Gaul,’ indicative of the discoverer’s nationality. Even after nearly a century and a half since its discovery, gallium continues to be a subject of curiosity and intrigue within the scientific community. The recent research not only enriches the existing body of knowledge but also serves as a reminder of how much remains to be explored within the periodic table.
As scientists delve deeper into the unique properties of gallium, it becomes evident that even the most seemingly established facts about this remarkable metal warrant scrutiny and reevaluation. The journey of understanding gallium’s atomic nature encapsulates a broader theme in scientific inquiry: the importance of questioning established norms and the insatiable pursuit of knowledge that drives innovation forward. The discoveries surrounding gallium are not just a testament to chemical science but also a harbinger of future breakthroughs that may reshape our technological landscape.

Leave a Reply