In the realm of biological sciences, the complexity of life often befuddles the most astute observers. One of the prevailing questions has been: How does matter, defined by its inanimate nature, achieve self-organization, embodying the very essence of life? A recent study led by Professor Anđela Šarić and her research team at the Institute of Science and Technology Austria (ISTA) sheds light on this enigma through the lens of bacterial cell division. Exploring a previously unexplored mechanism, the study reveals that misaligned protein filaments within bacteria ‘die’ to facilitate a vital ring structure necessary for cell replication. This remarkable discovery, documented in *Nature Physics*, introduces potential applications in synthetic biology and materials science.
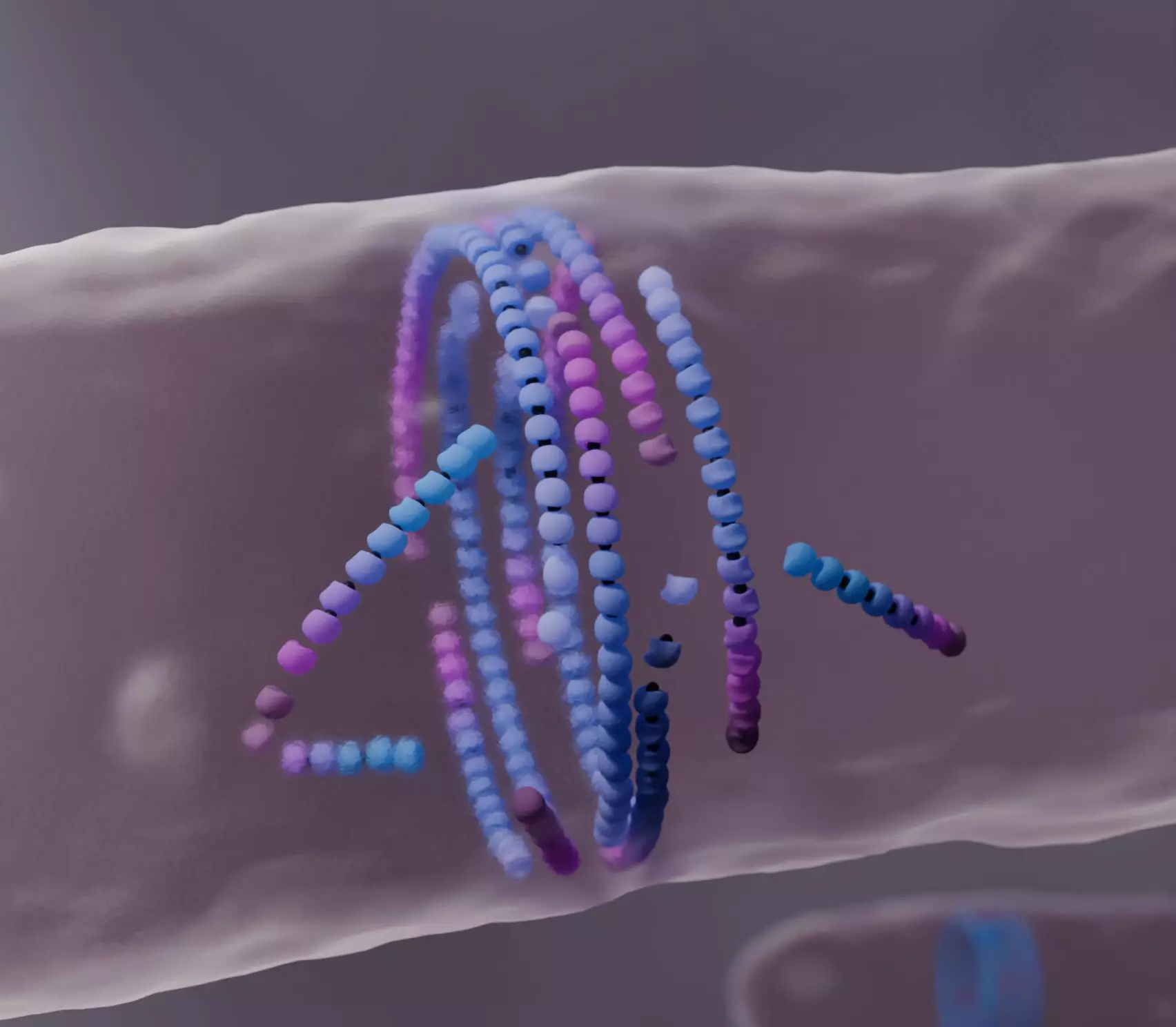
At the heart of this research lies the protein filament called FtsZ, a critical player in bacterial cell division. The FtsZ protein utilizes a process known as “treadmilling”—an intricate dance of growth and shrinkage that allows these filaments to dynamically self-assemble into a ring at the center of the dividing bacterial cell. This ring serves as a foundational structure for constructing the septum, the division wall that separates the emerging daughter cells. However, the physical principles guiding FtsZ self-assembly had long remained elusive, prompting researchers to dive deeper into the intricate behaviors of these active matter systems.
In their effort to understand how misalignment among FtsZ filaments could be beneficial, the Šarić group developed a sophisticated computational model. Their findings reveal a novel behavior: when misaligned filaments encounter obstacles, they do not simply stop but rather “die,” allowing for the reconfiguration into a more organized structure that favors the formation of the division ring. Such an approach marks a crucial shift from conventional perspectives of self-propelled biological systems, emphasizing the importance of filament turnover and assembly in the process of self-organization.
A unique aspect of this research was the collaboration between the Šarić team and experimentalists from different institutions, notably Seamus Holden’s group at the University of Warwick and Martin Loose’s team at ISTA. Their cross-disciplinary engagement was instrumental in validating the computational results observed in simulations. During a conference aptly named “Physics Meets Biology,” the researchers discovered a complementary synergy: while Holden provided imaging data outlining the life cycles of FtsZ filaments, Loose’s experimental observations in vitro substantiated the computational predictions. This collaboration exemplifies how collective efforts across scientific disciplines can yield powerful insights, further illuminating the complex interplay between theory and empirical evidence.
The implications of understanding FtsZ’s mechanistic role extend beyond the realm of microbiology. As Šarić points out, the ability of active matter to self-organize through energy-driven turnover opens avenues for engineering synthetic self-healing materials and artificial life forms. By mimicking the processes observed in bacterial cell division, synthetic researchers could design materials that dynamically adapt and recover from physical damage, thereby exhibiting lifelike qualities despite being non-living. The prospect of developing synthetic cells—structures that mimic the functionalities of biological cells—could revolutionize biotechnology and materials creation.
The groundbreaking research conducted by Šarić and her colleagues unveils critical mechanisms underpinning active matter self-organization during bacterial cell division. By focusing on the shocking behavior of FtsZ filaments and their tendency to ‘die’ for the sake of organization, the study redefines our understanding of self-assembly processes in biology. While fully comprehending the underlying principles of life remains a monumental challenge, collaborative efforts, such as those undertaken by the ISTA team, illuminate pathways forward—promising not only answers to fundamental questions but also potential applications in synthetic biology that could mimic the remarkable resilience and adaptability seen in nature. The quest for knowledge continues as researchers strive to unravel the intricate tapestry of life from the simplest components.

Leave a Reply