Helices play a pivotal role in various biological processes, primarily through their presence in proteins, which are essential for life. These coiled structures are not just visually appealing; they are fundamentally important to the shape and functionality of proteins. A helix is formed by the specific arrangement of amino acids, which are the basic building blocks of proteins. Understanding how these helical formations develop can lead to remarkable insights into protein behaviors and interactions within the human body.
Peptides, which are smaller versions of proteins, exhibit helical structures that arise from repeating sequences of amino acids. This repetition creates distinct patterns, imparting unique properties to the peptides. Chirality, a term used to describe the ‘handedness’ of structures, plays a vital role in how these helices are formed and how they function. The orientation and arrangement of amino acids can lead to new layers of chiral information within the molecule, thereby influencing their biological roles.
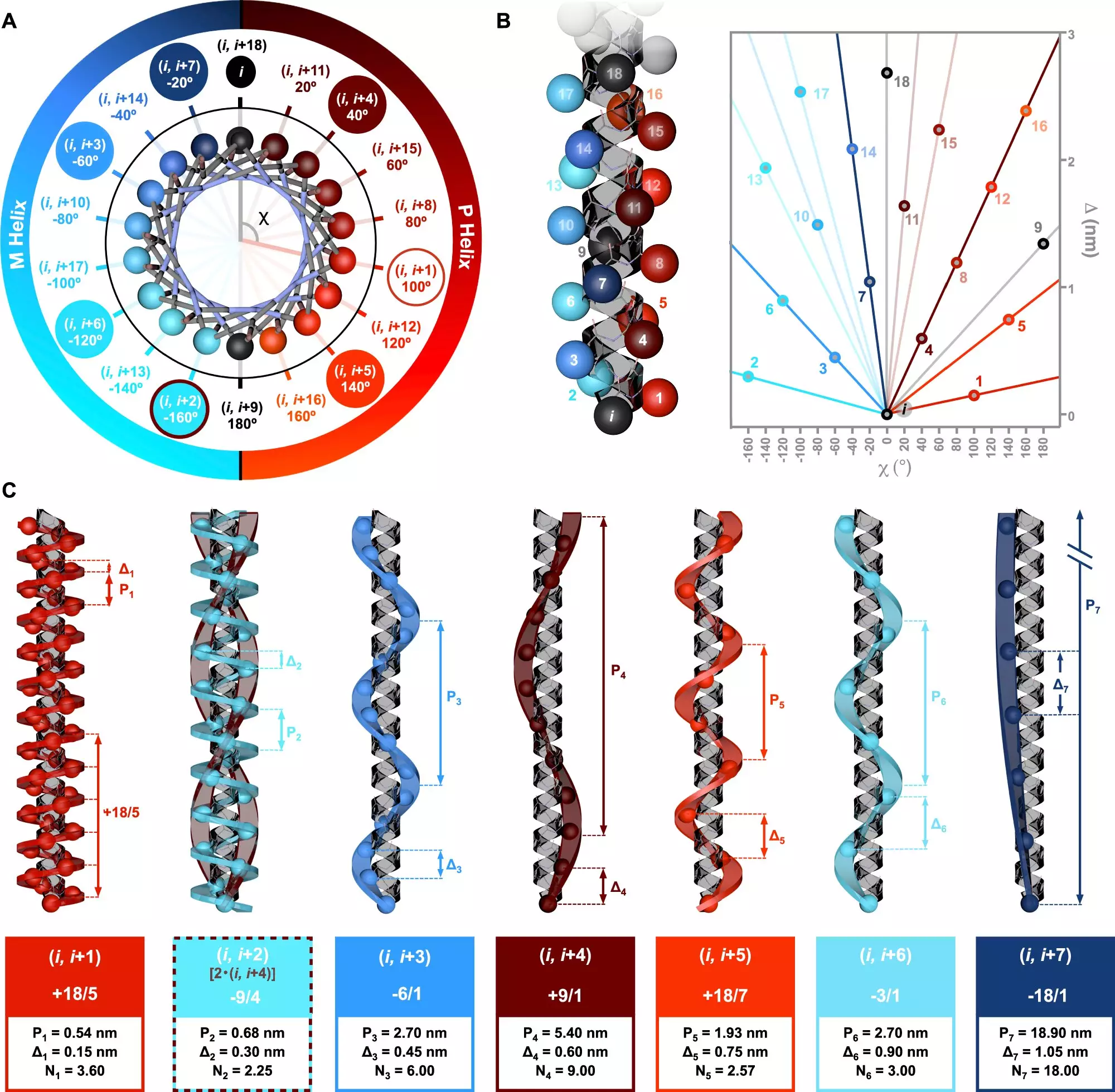
The significance of chirality in peptide structure has recently been explored in a groundbreaking study led by Dr. Julián Bergueiro at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS). Published in Nature Communications, this research sheds light on the intricate ways in which different amino acid sequences can create various helical configurations. For the first time, the authors introduced a symmetry model that elucidates the relationships between these structures, pushing the boundaries of our understanding in peptide chemistry.
The research employed advanced computational methods along with circularly polarized light spectroscopy to characterize the helical topologies of peptides. This innovative technique allowed the team to observe how these peptides interact with chiral light, thereby confirming the theoretical predictions regarding their structures. The results indicated that distinct amino acid arrangements yield unique helical forms, underscoring the complexity and variety within protein structures.
Dr. Bergueiro stated that this research could significantly impact our comprehension of protein functionalities, which are central to numerous biological processes. The ability to manipulate amino acid sequences would enable scientists to engineer specific topologies along polymer chains, opening up new avenues in macromolecular engineering. This flexibility in design could lead to the creation of tailored compounds, which have vast implications for medicine, biotechnology, and the development of biocompatible materials.
This study represents a significant milestone in the field of peptide chemistry, enhancing our understanding of how helical structures can be harnessed for various applications. The implications of being able to control the sequences of monomers not only pave the way for new therapeutic agents but also for advanced biomaterials that could interact more efficiently with biological systems. As research in this area continues to progress, we may soon witness revolutionary developments in the way we approach complex biological problems, ultimately benefiting healthcare, environmental sustainability, and materials science.
The exploration of helical structures in proteins reveals a layer of complexity that is critical to their function. By delving deeper into the chiral aspects of these molecules, researchers are not only expanding the body of knowledge in the field but also creating exciting new possibilities for innovative technologies in the future.

Leave a Reply