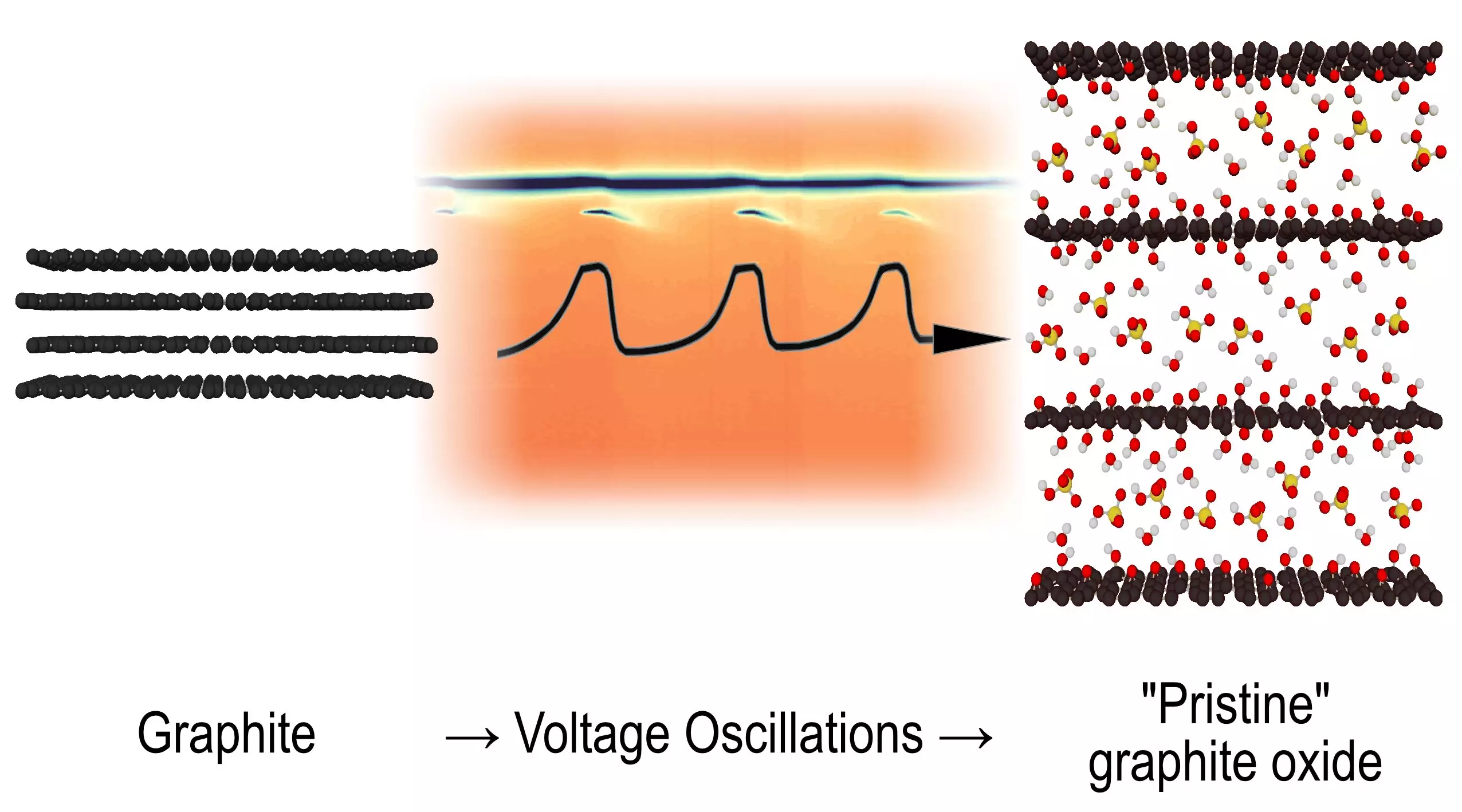
For decades, scientists have been captivated by the complex behaviors of chemical reactions. Among these enigmas is a peculiar reaction involving graphite, which has stymied researchers for an astonishing half-century. Recent breakthroughs from Umeå University have illuminated the mysterious transformation of graphite into graphite oxide during electrochemical oxidation, shedding light on intermediate structures and unveiling a novel phenomenon. This revelation not only broadens our understanding of electrochemistry but also challenges existing paradigms within the field.
The intricate mechanics behind the change from graphite to graphite oxide had long remained under wraps, primarily due to the elusive characteristics of the reaction. When graphite electrodes are subjected to voltage in a sulfuric acid solution, oscillations in voltage spontaneously emerge. While this behavior has been recognized for years, the step-by-step transformation process has largely eluded observation until now. Researchers from Umeå, employing advanced synchrotron methods, captured rapid X-ray diffraction scans that reveal revealing snapshots of the structural evolution throughout the oscillation cycles.
The transformation is not linear; rather, it unfolds in a rhythmic pattern reminiscent of oscillation. This cyclical process introduces intermediate structures that vanish and re-emerge, suggesting a deeper complexity at play. Alexandr Talyzin, a leading figure in this research, emphasizes this momentous discovery, stating that what began as a focused investigation has blossomed into a significant moment for fundamental chemistry.
Fascination surrounding oscillating reactions is not merely academic; it connects to various aspects of both chemistry and nature. These reactions, often demonstrated visually through distinct color changes in solutions, encapsulate the essence of dynamism in chemical processes. Understanding the underlying mechanisms is crucial not just for theoretical knowledge but also for practical applications in materials science, energy storage, and beyond.
The newly observed oscillating reaction provides a direct link to Ilya Prigogine’s foundational work, which won him the Nobel Prize in 1977. Prigogine’s research tackled the complexity of non-equilibrium thermodynamics and introduced the idea that order can arise from chaotic systems. Such insights are pivotal for comprehending how oscillating phenomena might emerge in various realms beyond electrochemistry.
Implications for Future Research
The results presented by Talyzin and his colleagues are not just a glimpse into the mechanics of graphite oxide formation; they signify a broader platform for future exploration. The distinct oscillating behavior of the reaction invites researchers to develop new theories and models that could expand upon existing knowledge. There is potential to explore reactions across different chemical landscapes, looking for similarities and patterns that mirror this newly identified framework.
The researchers articulated their aspiration for the scientific community, hinting at the possibility of uncovering additional examples of oscillating reactions. This outlook suggests a transformative era in chemical research, where understanding the scopes and limitations of current models could lead to fresh perspectives and innovative applications.
The recent findings from Umeå University mark a significant leap forward in the understanding of graphite oxidation and oscillating reactions in inorganic chemistry. By capturing rapid snapshots of a previously enigmatic process, scientists have not only unraveled a long-held mystery but also laid the groundwork for future inquiries into complex chemical behaviors. Such pioneering work underscores the delicate balance between chaos and order in chemical systems and invigorates the quest for knowledge across various disciplines within science. As research continues to evolve, we may witness a paradigm shift in the way chemical reactions are perceived, explored, and utilized, reinforcing the vital interplay between observation, analysis, and discovery in the intricate world of chemistry.

Leave a Reply